 Home |
FAQ |
Search |
Feedback
Home |
FAQ |
Search |
Feedback
 Home |
FAQ |
Search |
Feedback
Home |
FAQ |
Search |
Feedback
Why are fatty acids important?
What does CoA have to do with fatty acid metabolism?
In order to participate in any metabolic process, fatty acids must first be activated. They are activated by being joined in thioester linkage (R-CO-SCoA) to the -SH group of coenzyme A. The thioester bond is a high energy bond. This is apparently because resonance structures which can occur in esters with alcohols, and which stabilizes them, cannot occur in thioesters. The greater size of the sulfur atom (compared to the carboxyl oxygen) diminishes the stability of the resonance forms. If the structure has less resonance stability, the energy released upon their hydrolysis will be greater.
Why are some fatty acids considered essential?
Certain fatty acids of the omega - 3 and omega - 6 classes are required for the synthesis of prostaglandins and other physiological regulators. Our systems cannot introduce double bonds into those positions because of the specificity of our desaturases. Hence, these fatty acids must come from dietary sources.
What is the structure of a fatty acid?
Fatty acids are carboxylic acids attached to alkyl chains. The common fatty acids in mammalian systems are unbranched, and may have one or more double bonds. These double bonds are cis. Branched chain fatty acids and fatty acids containing aliphatic ring systems are known.
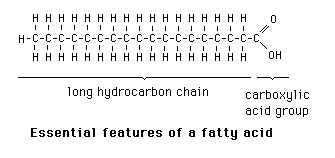
How are fatty acids named? How is the position of a double bond indicated? How does the omega nomenclature system work?
The most abundant fatty acids are generally called by their nonsystematic common names. Persons who will be working with these fatty acids will learn the common nomenclature whether they approve of it or not.
Positions of
double bonds are always designated by the number of the involved
carbon that is closest to the reference point. For example, in
the IUPAC system and the Delta abbreviation system, the reference
point (numbered 1) is the carboxyl carbon. Hence a double bond in
the 9-position, which is the same as a ![]() 9
double bond, is between the 9-carbon and the 10-carbon. In the
omega system the reference point is the last carbon in the chain,
farthest from the carboxyl carbon. An omega
- 3 (omega minus
three) double bond joins the third carbon from that end to the
fourth carbon from that end.
9
double bond, is between the 9-carbon and the 10-carbon. In the
omega system the reference point is the last carbon in the chain,
farthest from the carboxyl carbon. An omega
- 3 (omega minus
three) double bond joins the third carbon from that end to the
fourth carbon from that end.
Which end of the growing fatty acid is the new two carbon unit (from the malonyl group) added to?
To the carboxyl end. The acetyl CoA that started the process is thus farthest from the carboxyl group of the finished fatty acid.
How are fatty acids released from adipose tissue?
Fatty acids are released from adipose by hydrolysis of their stored form, triacylglycerol. Hydrolysis is initiated by activation of the hydrolytic enzyme, hormone sensitive lipase (HSL). HSL is a phospho-dephospho enzyme which is active in the phospho- form. Phosphorylation of HSL is stimulated by the hormones epinephrine, norepinephrine, cortisol and ACTH. These hormones bind to the surface of the adipocyte, where they activate adenyl cyclase, initiating a typical c-AMP-mediated phosphorylation cascade, terminating with phosphorylation of HSL. After release from adipocytes, unesterified fatty acids are transported in the blood bound to serum albumin to tissues such as liver, heart and muscle, where they are taken up and oxidized.
How are fatty acids activated?
Fatty acids are activated by reaction with CoA to form fatty acyl CoA. The reaction normally occurs in the endoplasmic reticulum or the outer mitochondrial membrane. This is an ATP-requiring reaction, yielding AMP and pyrophosphate (PPi). Different enzymes are specific for fatty acids of different chain length. Subsequent hydrolysis of the PPi by a pyrophosphatase draws the activation to completion.
How is fatty acyl CoA transported from the cytoplasm to the mitochondria for beta-oxidation?
Cytoplasmic fatty acyl CoA is converted to fatty acyl carnitine by carnitine acyl transferase (CAT I), an enzyme of the inner leaflet of the outer mitochondrial membrane. Fatty acyl carnitine is then trransported by an antiport in exchange for free carnitine to the inner surface of the inner mitochondrial membrane. There carnitine acyl transferase II (CAT II) reverses the process, producing fatty acyl CoA and carnitine. This shuttle mechanism is required only for longer chain fatty acids. Medium- and short chain fatty acids are carnitine-independent. They cross the mitochondrial membranes, and are activated in the mitochondrion.
What are the differences between fatty acid synthesis and beta-oxidation?
The two processes are superficially the reverse of one another. There are, however, several important differences, allowing for differential control, with one process inhibited while the other is stimulated.
| Synthesis | Beta-Oxidation | |
|---|---|---|
| cytoplasm | mitochondria | |
| multifunctional enzyme | separate enzymes | |
| NADPH | NAD and FAD | |
| Energy
requirement: 49 ATP equivalents |
Energy
yield: 33 ATP equivalents |
|
| Regulation: Acetyl CoA carboxylase |
Regulation: Acetyl CoA availability |
Where are ketone bodies made? Where are they used? What accounts for this difference?
Ketone bodies are synthesized in the liver mitochondria, which is where HMG CoA lyase is expressed. This is the enzyme that cleaves HMG CoA, forming acetoacetate and acetyl CoA. (In endoplasmic reticulum of liver and most other cells, in contrast, HMG CoA reductase is found, which converts HMG CoA to mevalonate, a precursor of the isoprenoids and the steroids.)
Ketone bodies are used by most aerobic tissues, particularly heart and muscle. The major enzyme for activating acetoacetate is the mitochondrial enzyme, succinyl CoA: 3-ketoacid CoA transferase. This enzyme is not found in the liver. Hence the liver is a net exporter of ketone bodies.
What regulates ketone body synthesis?
The primary regulator of ketone body synthesis is fatty acid availability. When hormonal conditions (e.g., high glucagon, low insulin) cause fatty acid concentration in the plasma to be high, malonyl CoA concentration in the liver cytoplasm is low (because acetyl CoA carboxylase is in the less active phosphorylated state). Fatty acyl CoA can enter the mitochondria at a high rate (because there is no inhibition of CAT I), and beta-oxidation proceeds at a high rate. The ensuing high mitochondrial concentration of acetyl CoA results in active ketone body synthesis.