SOPHOMORE REPRODUCTIVE ORGAN
SYSTEMS
STUDENT GROUPS
Group
A Group C Group E
Adams,
Jeremy Hall,
Robert Richins,
Janeen
Alimov, Victoria Hart,
Brandon Richins,
Michelle
Anderson,
David Hayes,
Harland Roper,
William
Baker,
Dawn He,
Chenyin Rose,
Robert
Baugh,
Andrew Helton,
Heather Rosenhan,
Branden
Bell,
John
Hinz, William Satterfield,
Trevor
Bentley,
Melissa Hughes,
Kevin Schmidt,
Benjamin
Bergvall,
Ethan Jensen,
Jasmin Sill,
Benjamin
Biggs,
Jeremy Johnson, Elizabeth Sjostrom, Christopher
Blamires,
Melissa Judd, Kyle Sybrowsky, Christian
Brady,
Matthew Keidel, Ann Sykes,
Christopher
Buchanan,
Marcus Keller, Merle Taylor, Cole
Burnett,
Tyler Kelly,
Elizabeth Turner,
Matthew
Campbell,
Aaron Keyser, Angela Tuttle, Marie
Cardenas,
Lilia Kurtz,
Angela Vargas,
Gabriela
Case,
Shanna Kwok,
Alvin Wachter,
Blake
Chan,
Christy Langelier,
Charles Waite, Aaron
Clark,
Randy Larsen,
Matthew Weiss, Aaron
Cole,
Jeromy Lawlor,
Cynthia Whittaker, Nathan
Coles,
Andrew Lodding,
Cynthia Winn, Darin
Cook,
Paul Lord,
Ken Wittwer,
Erica
Yee,
Candice
Group
B Group D
Cox,
Brady Mccandless,
Jeremy
Curtis,
Benjamin McIff,
Matthew
Dahle,
Nathan Mickelson,
Travis
Dodgion,
Christopher Miller,
Nathaniel
Donaldson,
Matthew Miller, Todd
Durrant,
Julia Mitchell,
Brian
Elijah,
Itoro Mohajer,
Arash
Ellison, Santina Moser,
Steve
Fischer,
Rachel Nackos,
Jeffrey
Ford,
Gregory Nguyen,
Tien
Frandsen,
Paul Nord, John
Garcia,
Josh Olsen,
Kathryn
Gardner, Kyle Patten,
Richard
Gonzalez, Sergio Payne,
Marielle
Green,
Layne Pennington,
David
Griner, Devan Petersen,
Sarah
Grunander,
Todd Pho, Thanh Lan
Guenter,
Jonathan Pippitt,
Karly
Gundersen,
Nancy Pradhan, Saphu
Hagn,
Emily Price,
Mary
Yonnet,
Gael Richards,
Nathan
LIFE CYCLE OF THE OVARY:
PUBERTY, THE MENSTRUAL CYCLE, AND MENOPAUSE
"A chicken is only an
egg's way of making another egg" --Samuel Butler
Objectives
- To describe normal and
abnormal puberty in males and females
- To understand the
hypothalamic, pituitary , ovarian, and uterine functions in the normal
menstrual cycle
- To understand the
normal transition from regular menstrual function to menopause and
describe menopausal symptoms
Definitions
Adrenarche: The increase in secretion of androgens by
the adrenal gland, occurring from about age 5 to age 20
Gonadarche: The initiation of production of significant
amount of sex steroids by the testis or
the ovary related to stimulation by gonadotropins
Puberty: The physical and biochemical changes
associated with maturation of the hypothalamic/pituitary/gonadal axis which
lead to the development of secondary sex characteristics and reproductive
function (usually the coordinated consequences of adrenarche and gonadarche)
Menopause: The final spontaneous menstrual period
(occurring at about 51 years of age in American women)
Climacteric: The period of transition from predictable
ovarian function through the postmenopausal years, a period marked by waning
ovarian function and dramatic decline in estrogen production
Perimenopause: The period before and after the final
menstrual period marked by fluctuating ovarian function (a period of about four
years on average)
Ontogeny of the
Reproductive System in Human: Fetal Life
Although
puberty occurs when increased gonadotropin secretions by the pituitary
stimulate the gonads, the stage has been set during fetal life. In males, the
earliest secretion of testosterone at 7 to 8 weeks gestation occurs independent
of gonadotropins and continues with stimulation of hCG. The
hypothalamic/pituitary axis completes development at about 20 weeks
gestation. After the development of the
portal system at 20 weeks, gonadotropins become sensitive to estrogen feedback
suppression and fall to undetectable levels.
Neonatal
Life
Immediately
after birth, the stimulatory effect of hCG on the male testis and the
suppressive effect of placental estrogens and progesterone on the pituitary and
hypothalamus are withdrawn, leading to a rapid rise in gonadotropins. The withdrawal
of placental hormones may actually lead to scant vaginal bleeding in females as
well as temporary nipple discharge. The subsequent pattern of gonadotropin
hormone levels and gonadal response differs in infant boys and girls. In girls,
there is a fall in estradiol levels in the first week of life and then a
gradual minimal rise that continues for one to two years. In boys, testosterone
levels rapidly decrease in the first week of life and then increase to pubertal
levels for two to four months before declining.
Childhood
The
period between infancy and puberty are marked by very low levels of
gonadotropins and gonadal steroids. Even in children without functioning gonads
(Turner's Syndrome, XO gonadal dysgenesis), the gonadotropins remain low suggesting
a profound suppression of the hypothalamic GNRH center. Classic experiments in
the rhesus monkey by Nobil (for which the Nobel Prize in medicine was awarded)
reveal that the administration of pulsatile GNRH to the prepubertal monkey can
initiate puberty.
Errors
in Suppression-Precocious Puberty
The
finding that the administration of pulsatile GNRH can initiate puberty and
experimentally induced lesions in the anterior hypothalamus in animals and can
cause precocious puberty suggests that there is an active "off"
center that suppresses the pulsatile release of GNRH. Accidents in nature in
humans (hypothalamic tumors, hydrocephalus, epilepsy) can lead to precocious
puberty in boys and girls. To date, no specific locus of suppression, which is
destroyed by tumors or turned "off" at puberty, has been found in
humans.
Normal
Puberty
Human
puberty is defined as the transition between the juvenile state and the mature
reproductive state when secondary sex characteristics develop and fertility is
achieved. It is composed of the relatively synchronous processes of adrenarche
and gonadarche. Adrenarche occurs usually one to two years before gonadarche
and is independent of gonadarche. Children without functioning gonads will
achieve adrenarche. Puberty includes the adolescent growth spurt, growth of
pubic and axillary hair in males and females, and specific secondary sex
characteristics for males and females.
The
age of puberty has been decreasing over the past several hundred years of
written documentation in Europe and the United States. Although the age of male
puberty is not as well documented as females, suggestions from Northern
European village records suggest that the age of menarche may have declined
from as late as 18 to the current 12.2 years. There are clear differences in
racial norms of puberty with African-Americans and Latinos achieving puberty at
a slightly earlier age on average than European-Americans.
Females
The
earliest manifestation of puberty in females is adrenarche. The rise in serum
DHEA and DHEAS may have no clinical signs or symptoms; therefore, the first
sign of puberty in females is usually defined as the initiation of breast buds.
The breast develops under unopposed low dose estrogen stimulation for about two
years before the first menses. During this time, pubic and axillary hair become
evident and there is a growth spurt. Weight gain occurs with increase in
height, but there is also an increase in body fat as distributed in the
breasts, mons pubis, hips and thighs. The vagina lengthens and becomes rugated,
and the labia majora and minora become thickened and rugated.
The
first menses occurs about two years after breast bud development and is usually
the result of fluctuating estrogens associated with follicle development
without ovulation. Ovulation usually occurs within six months from the first
episode of vaginal bleeding. The breast and pubic hair development as well as
vertical growth and fat deposition continue for several years after the first
menses.
Males
As
in females, puberty begins with adrenarche that also has limited clinical
manifestations in boys. The first clinical manifestation is testicular
enlargement, which begins at a mean age of 11.6 and is followed in the next two
years by pubic hair. Adult size and shape of the penis and scrotum is achieved
between ages 12 and 17 with an average of about 15 years of age, and pubic hair
completes development at about the same time.
The
testosterone effect on the vocal cords leads to the beginnings of voice
changing at an average age of 13, accompanied by the onset of spermatogenesis.
The growth spurt continues with 45 % of the adult skeletal mass acquired
between age 11 and age 18. Prior to puberty, males and females have similar
muscle mass; but by the end of puberty, the average male has more muscle mass than the average female.
The
emotional responses to the changes in gonadal steroid are poorly understood,
although all families and societies describe a marked change in pubertal
children with respect to their relationships with their parents, peers and
members of the opposite gender. Violent events by males increase dramatically
in adolescence, but whether this is a direct effect of gonadal steroids on
behavior or a function of the individual adolescent's character and societal
roles is not clear.
Errors
in Puberty (Delayed Puberty)
Delayed
puberty may be due to dysfunction of the hypothalamic/pituitary axis, end organ
failure, or may be idiopathic.
Constitutional delay of puberty may be due to chronic severe medical illness,
weight loss or malnourishment, or physical stress (including chronic strenuous
exercise).
Adrenarche
usually occurs, but gonadarche does not follow. Delayed puberty may also be due
to pituitary or hypothalamic tumors, pituitary failure, or congenital absence
of GNRH neurons.
Gonadal
failure in boys or girls may be due to chromosomal anomalies (Turner's
syndrome), exposure to high dose chemotherapy or radiation to the pelvis in
childhood, autoimmune or idiopathic. Adrenarche also still occurs (except in
those children with pituitary and subsequent adrenal failure), but development
of secondary sex characteristics does not follow. An evaluation of delayed
puberty should be evaluated in girls who have no evidence of breast development
by age 14 and in boys who have no evidence of genital growth by age 15.
The Normal
Menstrual Cycle
Overview
One
can analyze the menstrual cycle from many different points of view. The lay
person is primarily aware of episodic uterine bleeding, the more or less
regular interval between the bleeding episodes, and the interruption of the
cycles by pregnancy. The hypothalamus and the pituitary, however, orchestrate a
month-long interaction of the hypothalamic-releasing factors, pituitary gonadotropins,
and steroid hormones. In the ovary, the morphologic and endocrine events of
dominant follicle maturation and ovulation contrast sharply with the more
sedate background of relentless early follicle development and subsequent
atresia (only one follicle ovulates out of every 999 which initiate
development). Meanwhile, the endometrium sees and responds to the cyclic and
sequential appearances of estradiol and progesterone. The biochemist measures
the concentrations of the relevant hormones in plasma throughout the cycle and
wonders how these circulating hormones reflect or cause the key events in the
menstrual cycle. Thus, the view that a person, or an organ, has of the
menstrual cycle is highly relative to the position from which it is observed.
Interaction
of Hypothalamus, Pituitary, and Ovary
Circulating
concentrations of sex steroids and gonadotropins throughout the menstrual cycle
are depicted in Figure 1. It is logical to begin an analysis of the hormonal
interactions with the observation that as the corpus-luteum involutes after a
cycle in which conception has not occurred, pituitary FSH release is increased
in response to declining estrogen and progesterone concentration. The resulting
rise in circulating FSH stimulates follicle growth and induces activity of the
aromatase enzyme system necessary for
estradiol synthesis. This process of recruiting a cohort of follicles
from among which one will typically become dominant takes place by about the fifth
day of the average menstrual cycle. More intense gonadotropin stimulation
before this time in the cycle usually leads to multiple follicle maturations
such as the use of gonadotropins for the treatment of infertility and in-vitro
fertilization.
In
response to FSH stimulation, the responsive follicles secrete estradiol, which
feeds back to suppression FSH release from the pituitary. As depicted in Figure
1, the estradiol concentration continues to rise, ultimately in exponential
fashion, throughout the follicular phase of the menstrual cycle despite the
declining levels of FSH. The explanation of this phenomenon lies within the
micro environment of the ovarian follicle. By the last few days before
ovulation, virtually all of the ovarian estradiol secreted is produced by the
ovary, and primarily by the follicle destined to ovulate. The surge of
estradiol secretion at this time is responsible for the mid- cycle surge of LH,
a positive feedback of estradiol. In women, the amount of estradiol necessary
to produce a positive feedback effect on LH release is a concentration of 200
pg/ml or more sustained for about 50 hours. Long-term high concentrations of
estrogens lead to pituitary suppression (as with oral contraceptive pills).
Ovulation occurs about 29 to 39 hours after the LH surge begins.
After
ovulation, the corpus luteum secretes progesterone at the rate of about 25
mg/day, yielding serum concentrations of the hormone typically between 5 and 25
ng/ml. This rate of steroid production by the early corpus luteum is roughly
equal to the entire steroid output of both adrenal glands. In addition, the
corpus luteum also secretes estradiol and 17-hydroxyprogesterone, an
intermediate metabolite between progesterone and estrogen. After rupture and release of the ovum,
capillaries penetrate the granulosa layer, enabling the delivery of circulating
cholesterol, the necessary substrate for progesterone biosynthesis. In the face
of these levels of sex steroid secretion, FSH concentration declines even
further, whereas LH secretion levels plateau and is important in stimulation of
the corpus luteurn. If conception does not occur, the potent LH-surrogate, hCG,
does not arrive on the scene to sustain corpus luteum function. Through
sustained intra-ovarian processes of programmed cell death, the corpus luteum involutes
12 to 14 days after ovulation. Serum sex steroid concentrations fall, and
menstruation ensues.
Endometrial
Response During the Menstrual Cycle
Estradiol
is clearly a mitogen in the endometrium. At histologic analysis of endometrial
tissue, glandular mitoses are typically seen. An increased risk of
andenocarcinoma of the endometrium is associated with exposure over a period of
many years to significant amounts of estrogen, either ingested orally,
administered parenterally, or formed endogenously, typically by extraglandular
aromatization of circulating androgens. This stimulation, without the naturally
occurring progesterone from ovulation, or the administration of progestin, may
lead to a hyperplastic endometrium and potentially, to cancer. Mitoses are
almost never seen in endometrial specimens during the postovulatory phase of
the menstrual cycle, and the incidence of adenocareinoma of the endometrium in
premenopausal women with normal ovulatory function is nearly zero. This also
explains the protective effect of oral contraceptives against endometrial
cancer, as these medications always include a progestin.
Falling
progesterone in the secretary endometrium leads to the local production of
prostaglandin by the decidua (the part of the endometrium which is sloughed
each month). Prostaglandin causes
vasospasm of the spiral arterioles, and subsequent ischemia and sloughing of
the endometrium is what patients experience as a "periods.' The uterine
cramping associated with the normal ovulatory cycle is caused by this
prostaglandin's action and explains the effectiveness of prostaglandin
inhibitors (aspirin or ibuprofen) in the treatment of dysmenorrhea.


The End of
Reproductive Life in Women
Perimenopause
The
reliability of ovarian function, both hormonally and reproductively, peaks in
the mid-to-late twenties. Beginning in the early thirties, there is
epidemiologic evidence of a decline in fertility. By the mid-thirties, there
are subtle changes in the levels of FSH in the early follicular phase that
become more marked in the forties. These changes may not be reflected in
clearly noticeable changes in the experience of an individual woman's menstrual
cycle. As the mid forties arrive, there may be a shortening of the length of
the menstrual cycle that is a reflection of a declining pool of oocytes,
declining inhibin, rising FSH and earlier efforts at recruitment and ovulation
of the dominant follicle. The nature of these changes as perceived by an
individual woman will be very different from person to person.
The
perimenopause is defined as that period around the menopause that is marked by
unpredictable ovarian function and menstrual irregularity. Epidemiologic
studies of normal women suggest that this is a period of about four years
around the menopause although the variation from woman to woman is large. This
time is marked by unpredictable ovulation and periods of both higher and lower
than usual estrogen levels. Uterine bleeding may be more or less than
"usual" in flow and the timing of uterine bleeding is also
unpredictable.
There
are numerous physical and psychological phenomena attributed to this time of
reproductive life (mood swings, vasomotor flushes, sleep disturbances,
headaches, memory problems, decreased libido, urinary incontinence). It is not
clear which are related to fluctuations of ovarian function, which are related
to aging, and which are psycho-social responses to mid-life which may vary from
person to person and culture to culture.
Menopause
The
menopause is the retrospective diagnosis of the "final" spontaneous
menstrual period. Usually a woman in her fifties who has not had a period for
over a year may look back and note that her menopause" was on a specific
date of her last spontaneous period. The average age of menopause in American
women is 51. Various inherited and environmental factors influence the age of
menopause. Cigarette smoking, living at high altitude, exposure to some
chemotherapeutic agents, and hysterectomy tend to slightly lower the age of
menopause or final cessation of ovulation.
Climacteric
The
climacteric is a term used for the transitional period including the
perimenopause and the several years after the menopause. There are specific
symptoms that some women may experience which are directly attributable to
estrogen withdrawal (vasomotor flushes, urogenital atrophy), and there are some
long-term aging and disease processes which are worsened by estrogen withdrawal
(osteoporosis, coronary artery disease). There are number of other symptoms of
aging which may be worsened by estrogen withdrawal (arthritis symptoms,
cognitive function) but the evidence is not so clear.
The
postmenopausal ovary is still capable of producing substantial amounts of weak
androgens (ovarian stroma stimulated by menopausal levels of LH) that are
peripherally converted to estrogens.
Issues
in Hormone Replacement
The
eventual cessation of ovulation is a "normal" event in human
development. Until the last several hundred years, human life span was usually
less than 50 years of age. The existence of a population of women who
predictably lived well beyond the age of reproduction is new in human history.
Through epidemiologic studies in aging women, many of whom took estrogen
hormones for the treatment of vasomotor flushes, it was noted that long-term
estrogen users had a decreased incidence of complications of osteoporosis and
coronary artery disease. The health benefits and risks of estrogen therapy
after menopause have been continuously evaluated over the past 35 years, and
this therapy is now being subjected to prospective randomized trials. Recent
prospective randomized trials of initiating continuous estrogen and progestin
in older postmenopausal women did not show a health benefit with respect to
protection against coronary artery disease, demonstrated a very small increase
in the incidence of breast cancer and thromboembolic disease, and showed a
decrease in osteoporotic fractures and colon cancer in women who took
estrogen/progestin compared to placebo.
Observations
from women who had a uterus and took only estrogen after menopause revealed an
increased risk of uterine cancer. Unopposed estrogen stimulation of the uterus,
whether due to endogenous estrogens or estrogen therapy, causes endometrial
hyperplasia and potentially adenocarcinoma of the uterus. The intermittent
addition of progestational agents for 12 days each month causing endometrial
shedding eliminates this increased risk. For older women, the thought of
monthly periods is unattractive and is one of the major reasons for lack of
compliance in post- menopausal hormone therapy. Another concern is the
possibility of a small increase in the risk of breast cancer in long-term
estrogen users. Exogenous estrogens for the menopause may also carry a very
small increased risk of deep venous thrombosis and gallstone formation.
Formulations
for estrogens and progestins and combinations of both will change dramatically
in the years to come as clinical research develops methods and formulations which
protect the heart and bone but which do not stimulate the endornetrium or
breast (Selective Estrogen Receptor Modulators).
Male
Climacteric - Does It Exist?
The
search for a physiologic event in men that would correlate to the menopause in
women has been largely unsuccessful. The "male menopause" as a
definable gonadal event does not exist. Although the secretion of testosterone
gradually declines with advanced age (the rate after 40 about 1% per year) is
not enough to account for any decrease in libido or erectile function. Rather,
the problems associated with loss of desire or erectile dysfunction are related
to disease states or specific changes related to aging and not testosterone
levels, themselves. The concept of a gradual decline in adrenal androgenic
steroids (DHEA and DHEAS) which begins in the mid to late 20's and may lead to
some decrease in physical vigor and musculoskeletal flexibility has recently
received a great deal of press coverage. These hormones are readily available
at most super-markets and health food stores without a prescription. As DHEAS
levels in men decrease by 50% from 20 years of age to 50 years of age, there is
a great deal of interest in these hormones as a potential "fountain of
youth" for men. Limited prospective randomized studies suggest that the
administration of DHEAS in middle-aged men does increase lean body mass.
With
an aging population and the possibility of a generation of physically
incapacitated elderly men and women, the search for anabolic agents that will
maintain musculoskeletal strength has become more intense. Several studies on
the administration of 'growth hormone' in elderly men suggest that it may
increase lean body mass and strength in older men.
In
numerous cross-cultural studies of men and women, there does not appear to be a
well-defined entity called the "mid-life crisis." In both men and
women, there is no well-defined increase in major depression or major affective
disorders in mid-life. At the time of menopause women do have more concerns
about health and aging than do men of similar age. However, the concepts
of involutional melancholia, empty nest syndrome, and mid-life crisis do not exist as normative
events in the life cycle of men and women.
Summary
1. Puberty is the coordinated sequence of biochemical and physiologic events including adrenarche and gonadarche that result in the growth spurt of adolescence, development of secondary sex characteristics, and reproductive capacity.
2. The
CNS activation of puberty may occur prematurely (before the age of 8 in girls
or 9 in boys) or may be delayed (age 14 in girls and 15 in boys), often
indicating underlying medical disease.
3. The
cessation of predictable ovarian function occurs over several years. The
menopause is defined as the last spontaneous menstrual period.
4.
Estrogen therapy significantly
decreases hot flushes and vaginal atrophy and may substantially decrease the
risk of postmenopausal osteoporotic fractures.
Menopausal estrogen therapy for more that 5 years in women over 50 has
been associated with a small increase in the detection of breast cancer.
5. There
is no clear rapid decline in gonadal function in men as there is in women,
although there is a dramatic decline in adrenal androgens from their peak after
puberty to middle age. Whether this is reflected in decreased function is
unclear.
Bibliography and Suggested
Reading
Grumbach
MM, Styne AM. Disorders of Puberty in the Male and Female. Reproductive
Endocrinology, 3' ed., Yen and Jaffe eds, W B Saunders, Philadelphia, 1991
Grumbach
MM, Sizonenko PC, Aubert ML eds. Control of the Onset of Puberty. Williams and
Wilkins, Baltimore, 1990 (the "bible" on the control of puberty in
humans)
Mishell
DR. Menopause: Physiology and Pharmacology. Year Book Medical Publishers,
Chicago, 1987 (a nice review of menopause)
Speroff,
Case, and Glass. Clinical Gynecologic Endocrinology and Infertility, 5th ed.,
1994. Williams and Wilkins (the classic text on reproductive endocrinology with
very good chapters on puberty, the menstrual cycle, and menopause as well as
many other reproductive endocrine topics)
Yanovski
JA, Cutler GE. The Reproductive Axis: Pubertal Activation. Reproductive Endocrinology, Surgery, and
Technology. Adashi, Rock, Rosenwaks eds., Lippincott-Ravin, Philadelphia, 1996
FERTILIZATION, EARLY PREGNANCY AND ITS DISORDERS
Harry H.
Hatasaka, M.D.
Objectives
The student should be able
to:
1. Understand the process of ovulation, fertilization and implantation.
2. List the presumptive, probable and positive signs of pregnancy.
3. Understand the basis of pregnancy tests and their limitations.
Ovulation
The ovulation process is
important if subsequent fertilization is to take place. This is an exquisitely
timed phenomenon dependent on a host of hormonal interactions involving a
variety of endocrine glands. Tubal function must also be adequate or the ovum
will not be picked up by the fallopian tube to be fertilized within the
ampulla.
Fertilization
Following ovulation, the
ovum with its cumulus oophorus cells are picked up by the fimbria of the
fallopian tube. The ovum has now formed the first polar body. It remains in the
ampulla portion of the tube and is viable for about 18 to 24 hours. If
fertilization does not occur, the ovum disintegrates and is destroyed by the
tube. Sperm will remain viable in the female reproductive tract for about 48
hours, although this can be quite variable. Sperm present in the ampulla meet
the cumulus oophorus mass and penetrate by chemical and mechanical means to
reach the zona pellucida. One sperm penetrates the zona pellucida, the second
polar body is formed, and the nuclear material of the sperm enters the
vitelline membrane. The diploid chromosome number is re-established, and mitotic
cell division can now occur.
Implantation
After fertilization occurs,
the fertilized egg remains in the fallopian tube for about 72 hours. During
this time there are several cellular division, but the size of the fertilized
ovum does not increase. Around 72 hours the zona pellucida fragments and falls
away. The pre-implantation embryo enters the uterine cavity for 60 to 72 more
hours, and the central cavity begins to form. A definite cell mass is forined
on one side of the blastocyst by the time implantation occurs. The trophoblast
cells burrow into the endometrial stroma to form syncytiotrophoblast. Primitive
amniotic and chorionic cavities begin
to form, and a germ disk is recognizable soon after implantation.
Diagnosis of Pregnancy
Most women suspect pregnancy
before seeking confirmation. However, it is sometimes necessary to
differentiate pregnancy from other causes of uterine enlargement and/or
amenorrhea. The signs and symptoms are as follows:
1. Presumptive
a. Cessation of menses
(amenorrhea).
b. Breast changes.
c. Vaginal discoloration.
d. Skin pigmentation.
e. Morning sickness.
f. Perception of fetal
movements (quickening).
g. Urinary frequency. h.
Fatigue.
2. Probable
a. Abdominal enlargement.
b. Uterine and cervical
changes (shape, size, consistency).
c. Intermittent uterine
contractions.
d. Ballottement of fetus.
e. Palpation of fetal parts.
f. Positive hormonal (hCG)
tests.
3. Positive
a. Fetal heart tones heard
or recorded.
b. Fetal movements perceived
by examiner.
c. Fetus identified
ultrasonically or radiologically.
The diagnosis is
substantiated by the appearance of softening of the cervix on pelvic
examination (Goodell's sign), a purple hue of the vagina and cervix (Chadwick's
sign) and compressibility and softening of the isthmus (Hegar's sign) by six to
eight weeks' gestation. Abdominal signs of pregnancy appear somewhat later.
From 14 weeks, enlargement of the uterus is palpable abdominally. Fetal
movement is felt by 18 to 20 weeks (quickening), and fetal heart tones are
heard with the fetoscope slightly later. With the doppler, fetal life can be
confirmed much earlier (9 to 12 weeks) than with conventional auscultation
methods.
Pregnancy Tests
The biochemical test for
pregnancy has evolved from dependence on laboratory animals to rapid accurate
assays of human chorionic gonadotropin (hCG) produced by the
syncytiotrophoblast.
Pregnancy tests generally
available currently are enzyme immunoassays (E.I.A.) utilizing monoclonal antibodies specific for hCG,. thus avoiding
false positive reactions with luteinizing hormone. Serum or urine may be
tested, and both cost about the same and can be run in about ten minutes. It is
sensitive to about 25 mIU/mL, making it reliable soon after implantation which
occurs seven or eight days after ovulation. At approximately the time a woman
expects her menses to begin, her hCG concentration will be about 100 mIU/mL if
she is pregnant. Therefore commercial urine home pregnancy tests are generally
positive by that time. Home pregnancy tests are considered qualitative (yes
or no) tests as opposed to quantitative tests.
Serum radioimmunoassay beta
subunit (RIA-hCG-P) testing measures only beta subunit hCG. It is sensitive to
approximately 5 mIU/mL and is particularly useful for diagnosing pregnancy very
early. Serial quantitative RIA-hCG-P analyses are helpful in diagnosing ectopic
pregnancies, distinguishing viable pregnancies from non-viable ones and for
monitoring trophoblastic diseases (such as hydatidiform mole).
Differential Diagnosis
Errors may be caused by
uterine fibroids and ovarian cysts which may be confusing by their size. Other
sources of diagnostic error are premature menopause, obesity, and other
endocrine causes of amenorrhea. Pseudocyesis (a psychiatric condition where a woman
feels and fully believes she is pregnant when she is not) may be accompanied by
many of the subjective symptoms and signs of true pregnancy, but the pelvic
signs of pregnancy are absent and the laboratory tests are negative. Lastly,
ectopic or tubal pregnancy should always be kept in mind in any woman of
reproductive age who develops menstrual abnormalities and pelvic pain along
with symptoms of pregnancy.
Spontaneous Abortion
Definition: The natural termination of
pregnancy prior to the 20th week of gestation or with fetal weight less than
500 gm.
Clinical Classification:
1. Threatened Abortion: Uterine bleeding in early pregnancy, with or
without cramping.
2. Inevitable Abortion: Symptoms of threatened abortion plus the
physical finding of dilatation of the internal os of the cervix.
3. Incomplete Abortion: Passage of a portion of the products of
conception from the uterus.
4. Complete Abortion: Passage (grossly) of all of the products of
conception from the uterus.
5. Missed Abortion: Retention of the conceptus in the uterus for a clinically appreciable time after
death of the embryo or fetus.
6. Habitual Abortion: The usual criterion is three or more consecutive
abortions.
Incidence: Clinically recognizable
spontaneous abortion occurs in 15% to 20% of pregnancies, the majority
occurring in the first three months. It is probable that at least as many
abortions occur very early in pregnancy without recognition of the event.
Causes of Abortion:
A. Fetal factors (most common).
1. Developmental anomalies
in more than 60% of cases (Hertig).
2. Chromosome abnormalities
(22% in Carr's study).
B. Maternal factors (less common, but more often treatable).
1. Systemic diseases.
a. Infections transmitted to
the fetus (viral, bacterial, protozoal).
b. Febrile illness without
fetal infection.
c. Peritonitis secondary to
infection or surgery.
d. Hypertensive vascular
disease.
e. Severe metabolic
disorders (diabetes, thyroid dysfunction).
f. Chronic debilitating
disease states.
2. Inadequate progesterone production (corpus luteum or placenta) is
a definite probably infrequent cause.
3. Immunologic Factors -
Women expressing serum Lupus anticoagulant and anticardiolipin antibodies in
high titers are at increased risk of abortion (antiphospholipid syndrome).
4. Trauma - a rare factor.
5. Psychosomatic - suspected but unproven factor.
6. Uterine abnormalities.
a. Malformation, especially
septate uterus.
b. Myoma (submucous).
c. Intrauterine synechiae
(bands).
d. Incompetent cervix.
A uterine abnormality is particularly
suspect with repeated late abortion (second trimester).
Complications of Abortion
A. Hemorrhage - More common with late abortions. Continued heavy
bleeding indicates retained tissue (incomplete abortion).
B. Infection (septic abortion) seen most commonly with
criminally-induced abortion but may ensue in spontaneous or therapeutic
abortion. Septic shock may occur in severe instances.
C. If a missed abortion is retained beyond one month, thromboplastin
passage into the maternal circulation may result in a clotting disorder (DIC).
This risk is greater in late abortion.
Therapy
A. Threatened Abortion - no specific therapy is rational since the
majority of abortions result from failure of normal fetal development and the
fetus usually is dead by the time of onset of bleeding. Management is directed
toward avoiding the complications of infection or excessive blood loss.
Of all women who present uterine bleeding in early pregnancy, fewer
than half proceed to abortion.
B. Inevitable and incomplete abortion - the aim of therapy is prompt
evacuation of the uterus to prevent hemorrhage or infection.
1. Intravenous oxytocin infusion.
2. Removal of tissue with
sponge forceps and uterine curettage (suction or instrumental).
An exception in the
management of "inevitable" abortion is that of cervical incompetence.
In this condition painless dilatation of the cervix has occurred (without
bleeding) in the mid trimester. In this circumstance, a purse-string suture of
the cervix (cerclage) may succeed in retaining the pregnancy.
C. Complete Abortion: No further therapy is required, but the patient
must be observed closely for continued bleeding or evidence of infection. These
complications most often indicate that not all of the tissue has been passed.
D. Missed Abortion: Most missed abortions will evacuate spontaneously
and should then be evaluated for completion of the process. If uterine
evacuation is delayed beyond four weeks, intervention to empty the uterus
should be considered to prevent a
coagulation disorder.
Ectopic Pregnancy
A. Defined: Ectopic pregnancy refers to implantation of the zygote
outside the uterus or in an abnormal location within the uterus.
B. Incidence.
1. Varies widely from study
to study.
2. Probably dependent on
population base (Jamaica 1:28).
3. From 1:64 to 1:350, but
generally accepted at 1: 130.
4. Recently has shown
increasing frequency.
C. Mortality.
1. Felt to be responsible
for 10% of matemal deaths.
2. Approximate maternal
mortality: 1-2/1,000.
D. Etiology.
1. Chronic PID.
2. Tubal damage (previous
surgery, endometriosis).
3. Hormonal factors slowing
ovum transport.
4. Menstrual bleeding
(unsuppressed).
5. Tubal atony or spasm.
6. Blighted conceptus -
features of blighted ovum are seen twice as often in tubal pregnancies.
7. Developmental
abnormalities of the tube.
8. Extrinsic obstruction.
9. IUD usage.
E. Pathology: "Normal" conceptus but with pathologic site.
1. Uterine changes.
a. In first two months
uterus growth may be comparable to normal pregnancy due to the circulating
hormonal changes of early pregnancy.
b. Decidual changes.
c. Arias-Stella
("Sturgis-Arias-Stella"): Secondary to hyperstimulation by
progesterone and estrogen (occurs in 60%), suggestive of tubal ectopic
pregnancy.
2. Pathologic distribution
of nidation.
a. Uterine.
(1) Cervical - 1.5%
(2) Diverticular - rare.
(3) Uterine sacculation - more rare.
(4) Intramural.
(5) Angular.
(6) Cornual - 2%.
(7) Rudimentary horn.
b. Tubal - 95%.
(1) Interstitial.
(2) Isthmic.
(3) Ampullar (most common).
(4) Infundibular.
(5) Fimbrial.
c. Interligamentous.
d. Ovarian - 1:9,000 to
1:60,000.
e. Abdominal - 1: 15,000
live births.
F. Diagnosis.
1. Clinical history will
give greatest amount of useful information.
a. Clinical history -
negative history of amenorrhea in 25%.
b. Pain - most common
symptom - more than 90%.
c. Syncope - -33'%.
2. Physical exam.
a. Signs of hypovolemia -
3.3 )% heart rate - blood pressure.
b. Pelvic mass - 50%.
c. Pelvic pain - especially
with movement of cervix.
d. Temperature.
(1) May be subnormal with acute blood loss.
(2) May be elevated when patient stable (2%).
e. Diaphragmatic
irritation - 10%.
3. Lab data.
a. CBC with differential.
(1) Hct - Hbg: almost always low.
(2) Leukocytosis: 50% greater than 15,000/cu mm.
b. Pregnancy testing: almost
always positive with RIA or EIA tests.
c. Ultrasonography: A
gestational sac should be seen using a transvaginal ultrasound probe when the
serum quantitative PhCG exceeds 2,500 mIU/mL (even 1,000 at some centers) in a
normal intrauterine gestation. The inability to detect an ectopic pregnancy
ultrasonographically DOES NOT rule out the possibility of ectopic pregnancy.
4. Surgical Diagnostic
Options.
a. Culdocentesis: quick and
simple with extremely high correlation in ruptured ectopics (90% to 95%).
b. D & C.
(1) Only 20% will show decidual response.
(2) Questionable value.
c. Laparoscopy: especially
if diagnosis is only a suspicion.
G. Differential diagnosis.
1 . Ectopic pregnancy
2. Pelvic inflammatory
disease.
3. Abortion: threatened or
incomplete.
4. Ovarian pathology:
torsion, cyst.
5. Acute appendicitis.
H. Treatment.
1. Lab: CBC, ABO-Rh, cross
match, electrolytes, UA.
2. Stabilize patient.
3. Salpingectomy.
4. Ipsilateral oophorectomy
with ovarian involvement.
5. Conservative approach.
a. Resection.
b. Expression.
c. Evacuation.
d. Linear salpingostomy.
6. Contralateral tube.
7. Hysterectomy: criteria.
8. The Rh negative patient.
9. Medical treatment: single
dose IM methotrexate, hyperosmolar glucose.
I. Prognosis
1. Tubal pregnancy
interferes with future reproductive ability in 50% to 60%.
2. Recurrent tubal pregnancy
ranges from 7.7% to 20%.
Fertilization, Early
Pregnancy and Its Disorders
Major Take Home Points:
The
number one reason for amenorrhea in a woman of reproductive age is pregnancy.
The diagnosis of early pregnancy is not always
straightforward; clinicians from all disciplines must become expert in the
methods of diagnosing pregnancy.
The
most common disorder of early pregnancy is abortion in all its varied
presentations.
The
most life-threatening disorder of early pregnancy is ectopic pregnancy. High
suspicion for ectopic pregnancy should always be maintained for gynecologic
patients, and prompt diagnosis and therapy should be reflexive.
PROLACTIN:
PHYSIOLOGIC AND PATHOLOGIC ASSOCIATIONS
C. Matthew Peterson, M.D.
Objectives
1. To understand the release and control of prolactin
secretion and its actions both
physiologically and pathologically.
2. To understand the anatomy, differentiation, and
development of the breast and
the actions of
various endocrine factors resulting in lactation.
3. To appreciate the workup and treatment in a case of
hyperprolactinernia and its
treatment.
4. To recogonize the potential CNS abnormalities that may result in hyperprolactinemia.
Expectations
I would expect all students to know the following
definitions and take-home points. I have included additional materials and
references for those who desire greater than a superficial knowledge on the
subject. The test will come from the definitions and take home points.
Definitions
Prolactin: A product of the
anterior pituitary 199 amino acids with glycosylated and nonglycosylated forms.
It possesses a myriad of effects with the most noticeable being lactation. Its
secretion is inhibited by prolactin-inhibiting factor.
Prolactin-inhibiting factor (PIF): Inhibits the release of prolactin and is purported to be dopamine that is secreted by the tuberinfundibular neurons.
Lactation: The production of milk through the actions of prolactin on breast tissue to create polyamines, casein, lactose and phosphlipids.
Galactorrhea: The secretion
of milky fluid from the breast at times other than pregnancy.
Micro/macroadenoma of the
pituitary secreting prolactin: Small tumors usually located in the lateral
aspects of the pituitary that are surrounded by a pseudocapsule which contains
secretory granules of prolactin. Microadenomas are < I cm; macroadenomas are
> 1 cm. Hypotheses for their origin include reduced pituitary
dopamine concentrations and/or a vascular isolation of the adenoma cells.
Take-Home Points
Normal mammary development depends on a critical interplay of appropriate fat deposition, vascular supply, and hormone interactions. Estrogen stimulation of ductal development and progesterone induced development of alveolar growth and the modulating activities of estrogen, progesterone, growth hormone, insulin, cortisol, thyroid and parathyroid hormone with prolactin result in a functional gland. Dopamine, which is secreted by the tuberoinfundibular dopaminergic neurons into the portal hypophyseal vessels, is the primary prolactin-inhibiting factor. Lactation postpartum occurs when the inhibitory activity of progesterone is reduced through its more rapid clearance compared to prolactin.
Progesterone antagonizes the
alveolar cell prolactin receptor and inhibits lactation by:
Inhibiting
the upregulation of the prolactin receptor
Reducing
estrogen binding
Competing for
binding at the glucocorticoid receptor.
Galactorrhea occurs with:
Stimulation of the afferent limb of the
neuroendocrine arc
Decreased dopamine release or transport or binding
Autonomous
prolactin secretion
Hypothyroidism
Chronic renal failure
Hyperprolactinemia may cause anovulation through:
A reduction in granulosa cell number and FSH
binding
Inhibition of granulosa cell 17P estradiol
production by interfering with FSH action
Inadequate luteinization and reduced progesterone
The suppressive effects of prolactin on GnRH
pulsatile release.
The combination of
amenorrhea and galactorrhea is associated with hyperprolactinemia in two-thirds
of cases. In over one-third of women with hyperprolactinemia, a radiologic abnormality
consistent with an adenoma is found. A pituitary microadenoma (< 1 cm) or
hyperplasia is the cause of hyperprolactinernia in most patients with
hyperprolactinemia . Macroadenomas are larger than 1 cm. Well over 90% of
untreated microprolactinomas do not enlarge over a 4-6 year period of time.
Both microadenomas and macroadenomas (>1 cm) are monoclonal in origin. MRI is the optimal radiologic technique to
evaluate the sella/suprasellar region. Most patients with hyperprolactinemia
due to a microadenoma can be reassured that they have relatively benign
condition (pituitary microadenoma or release of pituitary stem cell growth
inhibition through activating or loss of function mutations in the pituitary
lactotroph) that requires only periodic monitoring. However, it is critical for
the physician to exercise vigilance and to consider the evaluation of other
potential etiologies, particularly sellar/suprasellar tumors. A TSH level
should be measured in all cases of hyperprolactinemia. Bromocriptine is the
mainstay of therapy for microadenomas and macroadenomas and in
hyperprolactinernia wihout evidence of an adenoma. While bromocriptine is the
best initial and potentially long-term treatment option for macroadenomas,
transsphenoidal surgery may be required if the adenoma is not responsive to
medical management. Breastfeeding is not contraindicated in the presence of
microadenomas or macroadenomas.
Chapter on Prolactin Disorders-Novacks Gynecology 2003 by C.
Matthew Peterson, M.D.
NOT REQUIRED UNDERLINED TEXT CONSIDERED SIGNIFICANT
Prolactin
Prolactin was first
identified as a product of the anterior pituitary in 1933 (168). Since that
time, it has been found in nearly every vertebrate species. The specific
activities of human prolactin (hPRL) have been further defined by the
separation of its activity from growth hormone (169) and subsequently by the
development of radioimmunoassays (170172). Although the initiation and
maintenance of lactation is the primary function of prolactin, many studies have
documented a significant role for prolactin activity both within and beyond the
reproductive system.
Prolactin Secretion
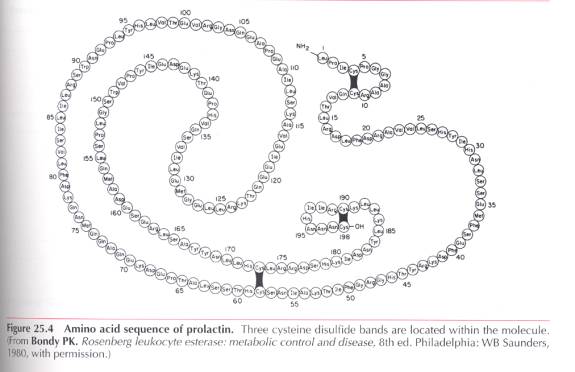
There are 199 amino acids
within hPRL with a molecular weight of 23,000 daltons
(Fig. 25.4). Although human growth hormone and placental lactogen
have significant lactogenic activity, they have only a 16% and 13% amino acid
sequence homology with prolactin, respectively.

In the basal state, three
forms are released: a monomer, a dimer, and multimeric species called little,
big, and big-big PRL, respectively (173-175). The two larger species can be
degraded to the monomeric form by reducing disulfide bonds (176). The
proportions of each of these prolactin species vary with physiologic,
pathologic, and hormonal stimulation (176-179). The heterogeneity of secreted
forms remains an active area of research. Overall, these studies indicate that little
prolactin (molecular weight [MW] 23,000) constitutes more than 50% of all
combined prolactin production (175,178,179) and is most responsive to
extrapituitary stimulation or suppression. The bioactivity and immunoreactivity
of little prolactin is influenced by glycosylation (180183). It appears that
the glycosylated form is the predominant species secreted, but the most potent
biological form appears to be the 23,000 MW nonglycosylated form of prolactin
(182). To some degree, the physical heterogeneity of prolactin may explain
the biologic heterogeneity of this hormone, but it further complicates the
physiologic evaluation of prolactins myriad effects.
In contrast to other anterior pituitary hormones, which are controlled by hypothalamic-releasing factors, prolactin secretion is primarily under inhibitory control mediated by dopamine. Multiple lines of evidence suggest that dopamine, which is secreted by the tuberoinfundibular dopaminergic neurons into the portal hypophyseal vessels, is the primary prolactin-inhibiting factor. Dopamine receptors have been found on pituitary lactotrophs (184), and treatment with dopamine or dopamine agonists suppresses prolactin secretion (185-190). The dopamine antagonist, metaclopramide, abolishes the pulsatility of prolactin release and increases serum prolactin levels (186,187,191). Interference with dopamine release from the hypothalamus to the pituitary routinely raises serum prolactin levels. Gamma-aminobutyric acid (GABA) and other neuropeptides may also function as prolactin-inhibiting factors (Table 25.6) (192195). Several hypothalamic polypeptides that increase prolactin-releasing activity are also listed (Table 25.6).
Table 25.6. Chemical Factors Modulating Prolactin Release and
Conditions That
Result in Hyperprolactinemia
Inhibitory factors
Dopamine
- Aminobutyric acid
Histidyl-proline diketopiperazine
Pyroglutamic acid
Somatostatin
Stimulatory factors
- Endorphin
17-Estradiol
Enkephalins
Gonadotropin=releasing hormone
Histamie
Serotonin
Substance P
Thyrotropin-releasing hormone
Vasoactive intestinal peptide
Physiologic conditions
Anesthesia
Empty sella syndrome
Idiopathic
Intercourse
Major surgery and disorders of chest wall (burns, herpes, chest
percussion)
Newborns
Nipple stiumulation
Pregnancy
Postpartum (nonnursing: days 1-7; nursing: with suckling)
Sleep
Stress
Postpartum
Hypothalamic conditions
Arachnoid cyst
Craniopharyngioma
Cystic glioma
Cysticercosis
Dermoid cyst
Epidermoid cyst
Histiocytosis
Neutrotuberculosis
Pineal tumors
Pseudotumor cerebri
Sarcoidosis
Suprasellar cysts
Tuberculosis
Pituitary conditions
Acromegaly
Addisons disease
Craniopharyngioma
Cushings syndrome
Hypothyroidism
Histiocytosis
Lymphoid hypophysitis
Metastatic tumors (especially of the lungs and breast)
Multiple endocrine neoplasia
Nelsons syndrome
Pituitary adenoma (microadenoma or macroadenoma)
Post-oral contraception
Sarcoidosis
Thyrotropin-releasing hormone administration
Trauma to stalk
Tuberculosis
Metabolic dysfunction
Ectopic production (hypernephroma, bronchogenic sarcoma)
Hepatic cirrhosis
Renal failure
Starvation refeeding
Drug conditions
Methyldopa
Antidepressants (amoxapine, imipramine,
amitriptyline)
Cimetidine
Dopamine antagonists (phenothiazines, thioxanthenes, butyrophenone,
diphenylbutylpiperidine, dibenzoxazepine,
dihydroindolone, procainamide,
metaclopramide)
Estrogen therapy
Opiates
Reserpine
Sulpiride
Verapamil
Hyperprolactinemia
When evaluating prolactin levels, physiologic alterations or conditions may result in transient as well as persistent elevations in prolactin levels. Drug-related and physiologic conditions resulting in hyperprolactinemia do not always require intervention.
Evaluation
Plasma levels of
immunoreactive prolactin are 527 ng/ml throughout the normal menstrual cycle.
Samples should not be drawn soon after the patient awakes or after procedures.
Prolactin is secreted in a pulsatile fashion with a pulse frequency ranging
from about 14 pulses per 24 hours in the late follicular phase to about nine
pulses per 24 hours in the late luteal phase. There is also a diurnal variation with the
lowest levels occurring in the midmorning.
Levels rise one hour after the onset of sleep and continue to rise until
peak values are reached between 5:00 and 7:00 AM (196,197). The pulse amplitude
of prolactin appears to increase from early to late follicular and luteal
phases (198200). Because of the variability of secretion and inherent
limitations of radioimmunoassay, an elevated level should always be rechecked.
This is preferably drawn midmorning and not after stress, venipuncture, breast
stimulation, or physical examination, which increases prolactin levels.
Prolactin and TSH
determinations are basic evaluations in infertile women. infertile men with
hypogonadism also should be tested. Likewise, prolactin levels should be
measured in the evaluation of amenorrhea, galactorrhea, amenorrhea with
galactorrhea, hirsutism with amenorrhea, anovulatory bleeding, and delayed
puberty (Fig. 25.5).


Physical Signs. Amenorrhea without
galactorrhea is associated with hyperprolactinemia in approximately 15% of
women (201-203). The cessation of
normal ovulatory processes attributed to elevated prolactin levels may be
related to the following gonadal and hypothalamic-pituitary effects: reduction in granuosa cell number and FSH
binding (204); inhibition of granulosa cell 17-b estradiol production by
interfering with FSH action (204-206); inadequate luteinization and reduced
progesterone (207-209); and the suppressive effects of prolactin on GnRH
pulsatile release, which may mediate most of the anovulatory effects (210-222).
Although isolated
galactorrhea is commonly considered indicative of hyperprolactinemia, prolactin
levels are within the normal range in nearly 50% of such patients (223225) (Fig. 25.5). In these cases, an earlier
transient episode of hyperprolactinemia may have existed, which triggered
galactorrhea. This situation is very similar to nursing mothers in whom milk
secretion, once established, continues despite normal prolactin levels. Repeat
testing is occasionally helpful in detecting hyperprolactinemia. Approximately
one-third of women with galactorrhea have normal menses. Conversely,
hyperprolactinemia commonly (66%) occurs in the absence of galactorrhea, which
may result from inadequate estrogenic or progestational priming of the breast.
In patients with both
galactorrhea and amenorrhea (including the syndromes described and named by Forbes, Henneman,
Griswold, and Albright, 1951; Argonz and del Castilla, 1953, and Chiari and
Frommel, 1985), approximately two-thirds will have hyperprolactinemia; and
in that group, approximately one-third will have a pituitary adenoma (226).
In anovulatory women, 310% with the diagnosis of polycystic ovarian disease
are noted to be hyperprolactinemic (227, 228) (Fig. 25.6).


In all cases of delayed
puberty, pituitary abnormalities, including craniopharyngiomas and adenomas,
must be considered. Additionally, the multiple endocrine neoplasia type 1
syndrome should be considered, particularly in patients with a family history
of multiple adenomas (229). Prolactinomas
are noted in approximately 20% of patients with multiple endocrine neoplasia
type 1 (MEN-1). MEN-1 gene is localized to chromosome 11q13
and appears to act as a constitutive tumor suppressor gene. An inactivating mutation results in the
tumor development. It is thought that
prolactinomas that present in patients with MEN-1 may be more aggressive than
sporadic prolactinomas (230). Prolactin
and TSH levels should be measured in all patients with delayed puberty.
Once an elevated prolactin
level is documented, the gynecologist must be familiar with neuroanatomy as
well as imaging techniques and their interpretation (see Chapter 7). Patients
can be reassured that hyperprolactinemia usually is associated with a
relatively benign condition (pituitary microadenoma or release of pituitary
stem cell growth inhibition through activating or loss of function mutations in
the pituitary lactotroph) that requires only periodic monitoring. However,
it is critical for the physician to exercise vigilance and to consider the
evaluation of other potential etiologies, particularly sellar/suprasellar
tumors. A TSH level should be measured in all cases of hyperprolactinemia (Figure 25.5)
Imaging Techniques
Prolactin levels in patients
with larger microadenomas and macroadenomas are usually higher than 100 ng/ml.
However, levels lower than 100 ng/ml may be associated with smaller
microadenomas and other suprasellar tumors that may be easily missed on a
coned-down view of the sella turcica. In patients with a clearly identifiable
drug-induced or physiologic etiology for hyperprolactinemia, scanning may not
be necessary. MRI imaging of the sella and pituitary gland with gadolinium
enhancement appears to provide the best anatomic detail (231) (Fig. 25.7). The cumulative radiation
dose from multiple CT scans may cause cataracts, and the coned-down views or
tomograms of the sella are very insensitive and likewise expose the patient to
radiation. The clinician must keep in mind that even modest elevations of
prolactin can be associated with microadenomas or macroadenomas, nonlactotroph
pituitary tumors, and other central nervous system abnormalities; and pituitary
imaging must be considered (Table 25.7).
For patients with hyperprolactinemia who desire future fertility, MRI is
indicated to differentiate a pituitary microadenoma from a macroadenoma as well
as to identify other potential sellar-suprasellar masses. Although infrequent,
when pregnancy-related complications of a pituitary adenoma occur, they occur
more frequently with macroadenomas (Table 25.7).
Well over 90% of untreated microprolactinomas do not enlarge
over a 4-6 year period of time. For
that reason, the argument that medical therapy will prevent a microadenoma from growing is false. While prolactin levels correlate with tumor
size, both elevations and reductions in prolactin levels may occur without any
change in size. If during follow-up a
prolactin level rises significantly or CNS symptoms (headache, visual changes)
are noted, repeat scanning may be indicated.


Hypothalamic Disorders
Dopamine was the first of many substances
demonstrated to be produced in the arcuate nucleus. Dopamine-releasing neurons
innervate the external zone of the median eminence. When released into the
hypophyseal portal system, dopamine inhibits prolactin release in the anterior
pituitary. Lesions that disrupt dopamine release can result in
hyperprolactinemia. Such lesions may arise from the suprasellar area, pituitary
gland, and infundibular stalk, as well as from adjacent bone, brain, cranial
nerves, dura, leptomeninges, nasopharynx, and vessels. Numerous pathologic
entities and physiologic conditions in the hypothalamic-pituitary region can
disrupt dopamine release and cause hyperprolactinemia (Table 25.7).


Pituitary Disorders Microadenoma
In over one-third of women
with hyperprolactinemia, a radiologic abnormality consistent with a
microadenoma is found. Release of pituitary stem cell growth inhibition via
activating and/or loss of function mutations result in cell cycle dysregulation
and are critical to the development of pituitary microadenomas and
macroadenomas. Microadenomas (<1 cm) are monoclonal in origin. Genetic mutations are thought to release
stem cell growth inhibition and result in autonomous anterior pituitary hormone
production, secretion and cell proliferation.
Additional anatomic factors which may contribute to adenoma formation
include reduced dopamine concentrations in the hypophyseal portal system,
vascular isolation of the tumor and/or both. Recently, the heparin-binding
secretory transforming gene (HST) has been noted in a variety of cancers as
well as in prolactinomas (232). Patients with
microadenomas (<1 cm) can generally be reassured of a benign course (233,234).
Both microadenomas and
macroadenomas (>1 cm) are monoclonal in origin. Pituitary prolactinomas or
lactotrope adenomas are sparely or densely granulated histologically. The sparsely granulated lactotrope adenomas
have trabecular, papillary or solid patterns.
Calcification of these tumors may take the form of a psammoma body or a
pituitary stone. The densely granulated
lactotrope adenoma is a strongly acidophilic tumor and appears to be more
aggressive than the sparsely granulated lactotrope adenoma. The unusual acidophil stem cell adenoma can
be associated with hyperprolactinemia with some clinical or biochemical
evidence of growth hormone excess.
Microadenomas rarely
progress to macroadenomas. Six large series of patients with microadenomas
reveal that with no treatment, the risk of progression for microadenoma to a
macroadenoma is only approximately 7% (235). Therapies include expectant,
medical, or, rarely, surgical therapy. All affected women should be advised to
notify their physician of chronic headaches, visual disturbances (particularly
tunnel vision consistent with bitemporal hemianopsia), and extraocular muscle
palsies. Formal visual field testing is rarely necessary.
Expectant Management. In women who do not desire
fertility, expectant management can be utilized for both microadenomas and
hyperprolactinemia without an adenoma if menstrual function remains intact.
Hyperprolactinemia-induced estrogen deficiency, rather than prolactin itself,
is the major factor in the development of osteopenia (236). Therefore, estrogen
replacement or oral contraceptives are indicated for patients with amenorrhea
or irregular menses. Patients with drug-induced hyperprolactinemia can also be
managed expectantly with attention to the risks of osteoporosis. In the absence
of symptoms, repeat imaging for microadenomas may be performed in 12 months to
rule out further growth of the microadenoma.
Medical Treatment. Ergot alkaloids are the
mainstay of therapy. In 1985, bromocriptine was approved for use in the U.S. to
treat hyperprolactinemia caused by a pituitary adenoma. The ergot alkaloids
increase dopamine levels, thus decreasing prolactin levels. Bromocriptine
decreases prolactin synthesis, DNA synthesis, cell multiplication, and tumor
growth. Bromocriptine treatment results
in normal prolactinemia or return of ovulatory menses in 80-90% of patients.
Because ergot alkaloids,
like bromocriptine, are excreted via the biliary tree, caution is required in
the presence of liver disease. The major adverse effects include nausea,
headaches, hypotension, dizziness, fatigue and drowsiness, vomiting, headaches,
nasal congestion, and constipation. Many patients tolerate the drug on the
following regimen: one-half tablet
every evening (1.25 mg), one-half
tablet morning and evening in the second week (1.25 mg q am and
q hs), and an increase of
one-half tablet every evening in the third week (1.25 mg q am, 2.5 mg q hs) and
every morning in the fourth week (2.5 mg twice a day). The lowest dose that
maintains the prolactin level in the normal range is continued. Pharmacokinetic studies show peak serum
levels occur three hours after an oral dose with a nadir at seven hours. There is little detectable bromocriptine in
the serum by 11-14 hours. Therefore,
twice-a-day administration is required.
Prolactin levels can be checked soon (6 to 24 hr) after the last
dose.
One rare, but notable
adverse effect of bromocriptine is a
psychotic reaction. Symptoms include
auditory hallucinations, delusional ideas and changes in mood that quickly
resolve after discontinuation of the drug (237).
Many investigators report no
difference in fibrosis, calcification, prolactin immunoreactivity, or the
surgical success in patients pretreated with bromocriptine compared to those
not receiving bromocriptine (238).
An alternative to oral
administration is the vaginal administration of bromocriptine tablets, which is
well tolerated (239). Cabergoline,
another ergot alkaloid, has a very long half-life and can be given orally once
per week. Its long duration of action
is attributable to slow elimination of pituitary tumor tissue, high affinity
binding to pituitary dopamine receptors, and extensive enterohepatic
recirculation.
Cabergoline, which appears
to be as effective as bromocriptine in lowering prolactin levels and in
reducing tumor size, has substantially fewer adverse effects. Very rare patients experience nausea and
vomiting with cabergoline; they may be treated with intravaginal caberoline
just as with bromocriptine. Although
caberogline appears to be safe during pregnancy; more extensive data regarding
the use of bromocriptine in pregnancy is available and is therefore preferred
in pregnant patients.
When bromocriptine or
cabergoline cannot be used, other medications such as pergolide or methergoline
may be used. In patients with a microadenoma who are receiving bromocriptine
therapy, a repeat MRI scan may be performed at 6-12 months after prolactin
levels are normal. Normal prolactin levels and resumption of menses should not
be considered absolute proof of tumor response to treatment. Further MRI scans
should be performed if new symptoms appear.
Discontinuation of bromocriptine therapy after 2-3 years may be attempted because some adenomas undergo hemorrhagic necrosis and cease to function.
Pituitary Disorders-Macroadenomas
Macroadenomas are pituitary
tumors that are larger than 1 cm in size. Bromocriptine is the best initial and
potentially long-term treatment option, but transsphenoidal surgery may be
required. Evaluation for pituitary hormone deficiencies may be indicated.
Symptoms of macroadenoma enlargement include severe headaches, visual field
changes, and, rarely, diabetes insipidus and blindness. After prolactin has
reached normal levels following ergot alkaloid treatment, a repeat MRI is
indicated within six months to document shrinkage or stabilization of the size
of the macroadenoma. This may be
performed earlier if new symptoms develop or if there is no improvement in
previously noted symptoms. Normalized
prolactin levels or resumption of menses should not be taken as absolute proof
of tumor response to treatment.
Medical Treatment. Macroadenomas treated with bromocriptine
routinely show a decrease in prolactin levels and size; nearly one-half show a
50% reduction in size, and another one-fourth show a 33% reduction after six
months of bromocriptine therapy. Because tumor regrowth occurs in over 60% of
cases after discontinuation of bromocriptine therapy, long-term therapy is the
rule.
After stabilization of tumor
size is documented, the MRI scan is repeated 6 months later and, if stable,
yearly for several years. Serum prolactin levels are measured every 6 months.
Because tumors may enlarge despite normalized prolactin values, a reevaluation
of symptoms at regular intervals (6 months) is required.
Surgical
Intervention Tumors that are unresponsive to bromocriptine or that cause
persistent visual field loss require surgical intervention. Some neurosurgeons have noted that a short 2-6 week course of preoperative bromocriptine
increases the efficacy of surgery in patients with larger adenomas (239).
Unfortunately, despite surgical resection, recurrence of hyperprolactinemia and
tumor growth are not uncommon. Complications of surgery include cerebral
carotid artery injury, diabetes insipidus, meningitis, nasal septal
perforation, partial or panhypopituitarism, spinal fluid rhinorrhea, third
nerve palsy, and recurrence. Periodic MRI scanning after surgery is indicated,
particularly in patients with recurrent hyperprolactinemia.
Metabolic Dysfunction
Occasionally, patients with
hypothyroidism exhibit hyperprolactinemia with remarkable pituitary enlargement
due to thyrotroph hyperplasia. These patients respond to thyroid replacement
with reduction in pituitary enlargement and normalization of prolactin levels
(240).
Hyperprolactinemia occurs in 2075% of women with chronic renal failure. Prolactin levels are not normalized through hemodialysis but are normalized after transplantation (241-244). Occasionally, women with hyperandrogenemia also have hyperprolactinemia. Elevated prolactin levels may alter adrenal function by enhancing the release of adrenal androgens such as DHEAS (245).
Drug-Induced Hyperprolactinemia
Numerous drugs interfere with dopamine secretion (Table 25.6). The same principles utilized in the management of pituitary microadenomas or hyperplasia can be applied in these situations. If discontinuation of the drugs is feasible, resolution of hyperprolactinemia is uniformly prompt.
Use of Estrogen in Hyperprolactinemia
In rodents, rapid pituitary
prolactin-secreting adenoma (prolactinoma) occurs with high-dose estrogen
administration (246). However, even conditions associated with high estrogen
levels, such as pregnancy, do not cause prolactinomas in humans. Indeed, pregnancy
may have a favorable influence on preexisting prolactinomas (247-24). Studies (249-251) and autopsy surveys
(228) indicate that estrogen administration is not associated with clinical,
biochemical, or radiologic evidence of growth of pituitary microadenomas or the
progression of idiopathic hyperprolactinemia to an adenoma status. For
these reasons, estrogen replacement or oral contraceptive use for
hypoestrogenic hyperprolactinemic patients secondary to microadenoma or
hyperplasia is appropriate.
Monitoring Pituitary Adenomas in Pregnancy
Prolactin-secreting
microadenomas rarely create complications during pregnancy. However, monitoring
of patients with serial gross visual field examinations and fundoscopic
examination is recommended. If persistent headaches, visual field deficits, or
visual or fundoscopic changes occur, MRI scanning is advisable. Because serum
prolactin levels are elevated throughout pregnancy, prolactin measurements are
of no value.
Although not recommended,
bromocriptine use during pregnancy in women with symptomatic (visual field
defects, headaches) microadenoma enlargement has resulted in resolution of
deficits and symptoms (253-256).
Pregnant women with previous
transsphenoidal surgery for microadenomas and/or macroadenomas may be
additionally monitored with monthly Goldman perimetry visual field testing.
Periodic MRI scanning may be necessary in women with symptoms or visual
changes. Bromocriptine has been used on a temporary basis to resolve symptoms
and visual field deficits in symptomatic macroadenoma patients to allow
completion of pregnancy before initiation of definitive therapy. Breastfeeding
is not contraindicated in the presence of microadenomas or macroadenomas (253256).
Chapter
References Prolactin Disorders
1.
Riddle
O, Bates RW, Dykshorn S. The preparation, identification and assay of
prolactin. A hormone of the anterior pituitary. Am J Physiol 1933;105:1916.
2.
Frantz AG, Kleinberg DL. Prolactin: evidence that it
is separate from growth hormone in human blood. Science 1970;170:7457.
3.
Lewis
UJ, Singh RNP, Sinha YN, VanderLaan P. Electrophoretic evidence for human
prolactin. J Clin Endocrinol Metab 1971;33:1536.
4.
Hwang
P, Guyda H, Friesen H. A radioimmunoassay for human prolactin. Proc Natl Acad
Sci USA 1971;68:19026.
5.
Hwang
P, Guyda H, Friesen H. Purification of human prolactin. J Biol Chem
1972;247:19558.
6.
Suh
HK, Frantz AG. Size heterogeneity of human prolactin in plasma and pituitary
extracts. J Clin Endocrinol Metab 1974;39:92835.
7.
Guyda
HJ, Whyte S. Heterogeneity of human growth hormone and prolactin secreted in
vitro: immunoassay and radioreceptor assay correlations. J Clin Endocrinol
Metab 1975;41:95367.
8.
Farkough
NH, Packer MG, Frantz AG. Large molecular size prolactin with reduced receptor
activity in human serum: high proportion in basal state and reduction after
thyrotropin-releasing hormone. J Clin Endocrinol Metab 1979;48:102632.
9.
Benveniste
R, Helman JD, Orth DN, McKenna TJ, Nicholson WE, Rabinowitz D. Circulating big
human prolactin: conversion to small human prolactin by reduction of disulfide
bonds. J Clin Endocrinol Metab 1979;48:8836.
10.
Jackson
RD, Wortsman J, Malarkey WB. Characterization of a large molecular weight
prolactin in women with idiopathic hyperprolactinemia and normal menses. J Clin
Endocrinol Metab 1985;61:25864.
11.
Fraser
IS, Lun ZG, Zhou JP, Herington AC, McCarron G, Caterson I, et al. Detailed
assessment of big prolactin in women with hyperprolactinemia and normal ovarian
function. J Clin Endocrinol Metab 1989;69:58592.
12.
Larrea
F, Escorza A, Valero A, Hernandez L, Cravioto MC, Diaz-Sanchez V. Heterogeneity
of serum prolactin throughout the menstrual cycle and pregnancy in
hyperprolactinemia women with normal ovarian function. J Clin Endocrinol Metab
1989;68:9827.
13.
Lewis
UJ, Singh RNP, Sinha YN, Vanderlaan WP. Glycosylated human prolactin.
Endocrinology 1985;116:35963.
14.
Markoff
E, Lee DW. Glycosylated prolactin is a major circulating variant in human
serum. J Clin Endocrinol Metab 1985;65:11026.
15.
Markoff
E, Lee DW, Hollingsworth DR. Glycosylated and nonglycosylated prolactin in
serum during pregnancy. J Clin Endocrinol Metab 1988;67:51923.
16.
Pellegrini
I, Gunz G, Ronin C, Fenovillet E, Peyrat JP, DeLori D, et al. Polymorphism of
prolactin secreted by human prolactinoma cells: immunological, receptor
binding, and biological properties of the glycosylated and nonglycosylated
forms. Endocrinology 1988;122:66774.
17.
Goldsmith
PC, Cronin MJ, Weiner RI. Dopamine receptor sites in the anterior
pituitary. J Histochem Cytochem
1979;27:12057.
18.
Quigley
ME, Judd SJ, Gilliland GB, Yen SSC. Effects of a dopamine antagonist on the
release of gonadotropin and prolactin in normal women and women with
hyperprolactinemic anovulation. J Clin Endocrinol Metab 1979;48:71820.
19.
Quigley
ME, Hudd SJ, Gilliland GB, Yen SSC. Functional studies of dopamine control of
prolactin secretion in normal women and women with hyperprolactinemic pituitary
microadenoma. J Clin Endocrinol Metab 1980;50:9948.
20.
DeLeo
V, Petraglia F, Bruno MG, Lanzetta D, Inaudi P, DAntona N. Different dopaminergic
control of plasma luteinizing hormone, follicle-stimulating hormone and
prolactin in ovulatory and postmenopausal women: effect of ovariectomy. Gynecol
Obstet Invest 1989;27:948.
21.
Lachelin
GCL, Leblanc H, Yen SSC. The inhibitory effect of dopamine agonists on LH
release in women. J Clin Endocrinol Metab 1977;44:72832.
22.
Hill
MK, Macleod RM, Orcutt P. Dibutyryl cyclic AMP, adenosine and guanosine
blockade of the dopamine, ergocryptine, and apomorphine inhibition of prolactin
release in vitro. Endocrinology 1976;99:16127.
23.
Lamberger
L, Crabtree RE. Pharmacologic effects in man of a potent long-acting dopamine
receptor agonist. Science 1979;205:11512.
24.
Braund
W, Roeger DC, Judd SJ. Synchronous secretion of luteinizing hormone and
prolactin in the human luteal phase: neuroendocrine mechanisms. J Clin
Endocrinol Metab 1984;58:2937.
25. Grossman A, Delitala G, Yeo
T, Besser GM. GABA and muscimol inhibit the release of prolactin from dispersed
rat anterior pituitary cells. Neuroendocrinology
1981;32:14550.
26.
Gudelsky GA, Apud JA, Masotto C, Locatelli V,
Cocchi D, Racagni G, et al. Ethanolamine-O-sulfate
enhances d-aminobutyric acid secretion
into hypophysial portal blood and lowers serum prolactin concentrations. Neuroendocrinology 1983;37:397-9.
27.
Melis
GB, Paoletti AM, Mastrapasqua NM, Strigini F, Fruzzetti F, Mais V, et al. The effects of the GABAergic drug, sodium
valproate, on prolactin section in normal and hyperprolactinemic subjects. J Clin Endocrinol Metab 1982;54:485-9.
28.
Melis
GB, Fruzetti F, Paoletti M, Mais V, Kemeny A, Strigini F, et al.
Pharmacological activation of d-aminobutyric acid-system
blunts prolactin response to mechanical breast stimulation in puerperal women.
J Clin Endocrinol Metab 1984;58:2015.
29.
Sassin
JF, Frantz AG, Weitzman ED, Kapen S. Human prolactin: 24-hour pattern with
increased release during sleep. Science 1972;177:12057.
30.
Sassin
JE, Frantz AG, Kapen S, Weitzman ED. The nocturnal rise of human prolactin is
dependent on sleep. J Clin Endocrinol Metab 1973;37:43640.
31.
Carandente
F, Angeli A, Candiani GB, Crosignani PG, Dammacco F, DeCecco L. Rhythms in the
ovulatory cycle. Prolactin Chronobiologia 1989;16:3544.
32. Pansini F, Bianchi A, Zito
V, Mollica G, Cavallini AR, Candini GC. Blood prolactin levels: influence of
age, menstrual cycle and oral contraceptives. Contraception
1983;28:2017.
33.
Pansini F, Bergamini CM, Cavallini AR, Bagni B,
Burghesani F, Agnello G, et al. Prolactinemia during the menstrual cycle. Gynecol
Obstet Invest 1987;23:1726.
34.
Bohnet
HG, Dahlen HG, Wuhjke W, Schneider HPG. Hyperprolactinemic anovulatory
syndrome. J Clin Endocrinol Metab 197;42:13243.
35.
Franks
S, Murray MAF, Jequier AM, Steele SJ, Nabarro JDN, Jacobs HS. Incidence and
significance of hyperprolactinemia in women with amenorrhea. Clin Endocrinol
1975;4:597607.
36.
Jacobs
HS, Hull MGR, Murray MAF, Frank S. Therapy oriented diagnosis of secondary
amenorrhea. Horm Res 1975;6:2687.
37.
McNatty
KP. Relationship between plasma prolactin and the endocrine microenvironment of
the developing human antral follicle. Fertil Steril 1979;32:4338.
38. Dorrington J, Gore-Lanton
RE. Prolactin inhibits oestrogen synthesis in the ovary. Nature
1981;290:6002.
39.
Cutie RE, Andino NA. Prolactin inhibits the
steroidogenesis in midfollicular phase human granulosa cells cultured in a
chemically defined medium. Fertil Steril 1988;49:6327.
40.
Adashi
EY, Resnick CE. Prolactin as an inhibitor of granulosa cell luteinization:
implications for hyperprolactinemia-associated luteal phase dysfunction. Fertil
Steril 1987;48:1319.
41.
Soto
EA, Tureck RW, Strauss JF III. Effects of prolactin on progestin secretion by
human granulosa cells in culture. Biol Reprod 1985;32:5415.
42.
Demura
R, Ono M, Demura H, Shizume K, Oouchi H. Prolactin directly inhibits basal as
well as gonadotropin-stimulated secretion of progesterone and 17 b-estradiol in the human ovary. J Clin
Endocrinol Metab 1985;54:124650.
43.
Boyar
RM, Kapen S, Finkelstein JW, Perlow M, Sassin JF, Fukushima DK, et al.
Hypothalamic-pituitary function in diverse hyperprolactinemic states. J Clin
Invest 1974;53:158898.
44.
Bohnet
HG, Dahlen HG, Wuttke W, Schneider HPG. Hyperprolactinaemic anovulatory
syndrome. J Clin Endocrinol Metab 1976;42:13244.
45.
Franks
S, Murray MAF, Jequier AM, Steele SJ, Nabarro JDN, Jacobs HS. Incidence and
significance of hyperprolactinemia in women with amenorrhea. Clin Endocrinol
(Oxf) 1975;4:597607.
46.
Moult
PJA, Rees LH, Besser GM. Pulsatile gonadotrophin secretion in
hyperprolactinaemic amenorrhoea and the response to bromocriptine therapy. Clin
Endocrinol 1982;16:15362.
47.
Buckman
MT, Peake GT, Srivastava L. Patterns of spontaneous LH release in normo- and
hyperprolactinaemic women. Acta Endocrinol 1981;97:30510.
48.
Aono
T, Miyake A, Yasuda T, Kolke K, Kurachi K. Restoration of oestrogen positive
feedback on LH release by bromocriptine in hyperprolactinemic patients with
galactorrhea-amenorrhea. Acta Endocrinol 1979;91:591600.
49.
Travaglini
P, Ambrosi B, Beck-Pecoz P, Elli R, Rodena M, Trotto G.
Hypothalamic-pituitary-ovarian function in hyperprolactinemic women. J Clin
Invest 1978;1:3945.
50.
Glass
MR, Shaw RW, Butt WR, London DR. An abnormality of oestrogen feedback in
amenorrhea galactorrhea. BMJ 1975;111:2745.
51.
Koike
K, Aono T, Tsutsumi H, Miyake A, Kurachi K. Restoration of oestrogen-positive
feedback effect on LH release in women with prolactinoma by transsphenoidal
surgery. Acta Endocrinol 1982;100:4928.
52.
Quigley
ME, Judd SJ, Gilliland GB, Yen SSC. Effects of a dopamine antagonist on the
release of gonadotropin and prolactin in normal women with hyperprolactinemic
anovulation. J Clin Endocrinol Metab 1979;48:71820.
53.
Boyar
RM, Kapen S, Finkelstein JW, Parlow M, Sassin JF, Fukushima DK.
Hypothalamic-pituitary function in diverse hyperprolactinemic states. J Clin
Invest 1974;53:1588.
54.
Rakoff
J, VandenBerg G, Siler TM, Yen SSC. An integrated direct functional test of the
adenohypophysis. Am J Obstet Gynecol 1974;119:35868.
55.
Zarate
A, Jacobs S, Canales ES, Schally AV, Cruz A, Sorra J, et al. Functional
evaluation of pituitary reserve in patients with the amenorrhea-galactorrhea
syndrome utilizing luteinizing hormone-releasing hormone (LH-RH), L-dopa and
chlorpromazine. J Clin Endocrinol Metab 1973;37:855.
56.
Kleinberg
DL, Noel GL, Frantz AG. Galactorrhea: a study of 235 cases, including 48 with
pituitary tumors. N Engl J Med 1977;296:589600.
57.
Tolis
G, Somma M, Van Campenhout J, Friesen H. Prolactin secretion in 65 patients
with galactorrhea. Am J Obstet Gynecol 1974;118:91101.
58.
Boyd
AE III, Reichlin S, Tuskoy RN. Galactorrhea-amenorrhea syndrome: diagnosis and
therapy. Ann Intern Med 1977;87:16575.
59.
Schlechte
J, Sherman B, Halmi N, Van Gilder J, Chapler FK, et al. Prolactin-secreting
pituitary tumors. Endocr Rev 1980;1:295308.
60.
Minakami
H, Abe N, Oka N, Kimura K, Tamura T, Tamata T. Prolactin release in polycystic
ovarian syndrome. Endocrinol J 1988;35:30310.
61.
Murdoch
AP, Dunlop W, Kendall-Taylor P. Studies of prolactin secretion in polycystic
ovary syndrome. Clin Endocrinol 1986;24:16575.
62.
Lythgoe
K, Dotson R, Peterson CM. Multiple endocrine neoplasia I presenting as primary
amenorrhea. A case report. Obstet Gynecol 1995;86:6836.
63.
Burgess
JR, Shepher JJ, Parameswaran V, et al.
Spectrum of pituitary disease in multiple endocrine neoplasia type 1
(MEN-1); clinical, biochemical, and radiologic features of pituitary disease in
a large MEN-1 kindred. J Clin Endocrinol
Metab 1996;81:2642-6.
64.
Bohler
HCL Jr, Jones EE, Briner ML. Marginally elevated prolactin levels require
magnetic imaging and evaluation for acromegaly. Fertil Steril 1994;61:116870.
65.
Gonsky
R, Herman V, Melmed S et al.
Transforming DNA sequences present in human prolactin-secreting
pituitary tumors. Mol Endocrinol
1991;5:187-95.
66.
Sisan
DA, Sheehan JP, Sheeler LR. The natural history of untreated
microprolactinomas. Fertil Steril 1987;48:6771.
67.
Schlechte
J, Dolan K, Sherman B, Chapler F, Ulciano A. The natural history of untreated
hyperprolactinemia: a prospective analysis. J Clin Endocrinol Metab 1989;4128.
68.
Weiss
MH, Teal J, Gott P, et al. Natural
history of microprolactinomas: six-year
follow-up period. Neurosurgery
1983;12:180-3.
69.
Klibanski
A, Biller BMK, Rosenthal DI, Schoenfeld DA, Saxe V. Effects of prolactin and
estrogen deficiency in amenorrheic bone loss. J Clin Endocrinol Metab
1988;67:12430.
70.
Turner
TH, Cookson JC, Wass JAH, et al.
Psychotic reactions during treatment of pituitary tumors with dopamine
agonists. GMJ 1984;289:1101-3.
71.
Weiss
MH, Wycoff RR, Yadley R, et al.
Bromocriptine treatment of prolactin-secreting tumorssurgical
implications. Neurosurgery
1983;12:640-2.
72.
Katz
E, Weiss BE, Hassell A, Schran H, Adashi EY. Increased circulating levels of
bromocriptine after vaginal compared to oral administration. Fertil Steril
1991;55:882.
73. Abram M, Brue T, Marange I,
Girard N, Guibout M, Jaquet P. Pituitary tumor syndrome and hyperprolactinemia
in peripheral hypothyroidism. Ann Endocrinol (Paris)
1992;53:21523.
74.
Chirito
E, Bonda A, Friesen HG. Prolactin in renal failure. Clin Res 1972;20:423.
75.
Nagel
TC, Freinkel N, Bell RH, et al. Gynecomastia, prolactin, and other peptide
hormones in patients undergoing chronic hemodialysis. J Clin Endocrinol Metab
1973;36:42832.
76.
Olgaard
K, Hagen C, McNeilly AS. Pituitary hormones in women with chronic renal
failure: the effect of chronic haemo- and peritoneal dialysis. Acta Endocrinol
1975;80:23746.
77.
Hagen
C, Olgaard K, McNeilly AS, Fisher R. Prolactin and the pituitary-gonadal axis
in male uremic patients on regular dialysis. Acta Endocrinol 1976;82:2938.
78.
Thorner
MO, Edwards CRW, Hanker JP. Prolactin and gonadotropin interaction in the male.
In: Troen P, Nankin H, eds, The Testis in Normal and Infertile Men. New York:
Raven Press, 1977:35166.
79.
Lloyd
RV. Estrogen-induced hyperplasia and neoplasia in the rat anterior pituitary
gland; an immunohistochemical study. Am J Pathol 1983;113:198206.
80. Scheithauer BW, Sano T,
Kovacs KT, Young W, Ryan N, Randall RV. The pituitary gland in pregnancy: a
clinico-pathologic and immunohistochemical study of 69 cases. Mayo Clin Proc
1990;65:46174.
81. Well C. The safety of bromocriptine in hyperprolactinemic female
infertility; a literature review. Curr Med Res Opin 1986;10:17295.
82.
Shy
KK, McTiernan AM, Daling JR, Weiss NS. Oral contraceptive use and the
occurrence of pituitary prolactinoma. JAMA 1983;249:22047.
83.
Corenblum
B, Taylor PJ. Idiopathic hyperprolactinemia may include a distinct entity with
a natural history different from that of prolactin adenomas. Fertil Steril
1988;49:5446.
84.
Corenblum
B, Donovan L. The safety of physiological estrogen plus progestin replacement
therapy and with oral contraceptive therapy in women with pathological
hyperprolactinemia. Fertil Steril 1993;6713.
85.
Scheithauer
BW, Kovacs KT, Randall RV, Ryan N. Effects of estrogen on the human pituitary:
a clinicopathologic study. Mayo Clin Proc 1989;64:107784.
86.
Krupp
P, Monka C. Bromocriptine in pregnancy: safety aspects. Klin Wochenschr 1987;65:8237.
87.
Raymond
JP, Golstein E, Konopka P. Follow-up of children born of bromocriptine-treated
mothers. Horm Res 1985;22:23946.
88.
Ruiz-Velasco
V, Tolis G. Pregnancy in hyperprolactinemic women. Fertil Steril
1984;41:793805.
89.
Turkalj
I, Braun P, Krupp P. Surveillance of bromocriptine in pregnancy. JAMA
1982;247:1589
NORMAL MATERNAL PHYSIOLOGY:
IMPLICATIONS FOR PRENATAL CARE
Objectives
1. List pertinent normal physiologic changes in the maternal
cardiovascular, respiratory, renal, hematologic, gastrointestinal, and
reproductive systems.
2. Describe the implications for these changes for normal and
abnormal pregnancies.
3. List the
nutritional requirements for calories, protein, iron, calcium, and folic add
for a normal pregnancy in a healthy gravid woman.
4. Describe the medical evaluation at the first prenatal visit and
then subsequent visits for a normal pregnant woman.
5. List at least five routine laboratory tests obtained early in
pregnancy and the rationale for each.
Definitions
1. Dilutional anemia of pregnancy: lower hematocrits are
seen in pregnancy because the expansion of plasma volume is greater than the
increase in red blood cell mass
2. Hypercoagulable state of pregnancy: increased
predilection for pregnant women to have clotting episodes
3. MSAFP (Maternal serum alpha-fetoprotein): Screening test
of maternal blood done in the early second trimester to screen pregnant women
for fetal anomalies and chromosomal abnormalities
4. Estimated delivery date (EDD): the estimated date of
delivery based on either dating or ultrasound parameters
5. Bacterial vaginosis: a bacterial infection of the vagina
associated with preterm labor and birth
6. Glucola: a screening test performed on maternal blood for
gestational diabetes
7. Rhogam: an antibody preparation of anti-Rh factor given
to Rh (-) women to prevent Rh isoimmunization
8. Neural tube defect (NTD): an abnormality in closure of
the neural tube, resulting in a spectrum of anomalies from anencephaly (no
cranium or cerebrum) to spina bifida
9. Intrauterine growth restriction (IUGR: pathological condition of abnormal
placentation resulting in an undergrown fetus
10. Small-for-gestational age (SGA): the lower 10% of
birthweights
11. Large-for-gestational age (LGA): the upper 10% of
birthweights
12. Macrosomia: an
abnormally large infant (usually > 4000 gm)
I. Introduction
The primary goal of prenatal care is to deliver a healthy
term infant without impairing the
mothers health and to identify and optimally treat the
high-risk parturient.
II. Pertinent
Changes in Normal Maternal Physiology
A. Cardiovascular system
1. Cardiac (see table I)
a. Cardiac output increases about 30-50%
(from 4.5 to 6.0 L/min, see figure 1)
b. Stroke volume increases about 10 to
15%
c. Pulse increases about 15-20 bpm
d.
Systolic ejection murmur and S3 gallop is common (about 90% of
pregnant women)
2. Blood pressure
a. Peripheral vascular resistance falls
b.
There is normally a fall in BP during the second trimester (5-10 mmHg systolic,
10-15 mmHg diastolic), and then returns to normal during the third trimester
Pertinence: Many of the effects of the
altered cardiovascular system mimic heart failure (edema, gallops, dyspnea,
distended neck veins, abnormal cardiac silhouette on CXR, EKG changes).
B. Respiratory system (see figure 2)
1. Unchanged: respiratory rate, vital capacity,
inspiratory reserve volume
2. Decreased: functional residual capacity (by 20%),
expiratory reserve volume (by 20%), residual volume (by 20%), total lung
capacity (by 5%)
3. Increased: inspiratory capacity (by 5%), tidal
volume (by 30-40%)
4. Arterial blood gasses: pH= 7.44, pC02=30,
bicarbonate=20-25, pO2=>100
Pertinence: A normal pregnant woman has
a compensated respiratory alkalosis and a diminished pulmonary reserve.
C. Renal system
1. Anatomic: increase in kidney size and weight,
ureteral dilatation (Right > left), bladder becomes an intra-abdominal organ
2. Hemodynamics:
a. GFR increases 50%, renal plasma flow increases by
75%
b. Creatinine clearance increases to 150-200 cc/min
3. Metabolic changes
a. BUN and serum creatinine decreases by about 25%
b. Plasma osmolarity decreases about 10 mOsm/kg H20
c. Increase in tubular reabsorption of sodium
d. Marked increase in renin and angiotensin levels,
but markedly reduced vascular sensitivity to their hypertensive effects
e. Increase in glucose excretion
Pertinence: Pregnant women are more
prone to pyelonephritis and bladder rupture during abdominal trauma.
D. Hematologic System
1. Plasma volume and RBC mass (see figure 3)
a. Plasma volume increases by about 50%
b. RBC volume increases by about 30%
c. The result: the "dilutional anemia of
pregnancy", such that the mean hemoglobin during pregnancy is about 11.5
g/dl
2. WBC and platelets
a. WBC count increases during pregnancy
b. Platelet count decreases, but stays within normal
limits
3. Coagulation system: pregnancy as a
"hypercoagulable state"
a. Increased levels of fibrinogen,
factor VII-X
b . The placenta produces a plasminogen activator
inhibitor
Pertinence: Blood loss is well-tolerated
during labor, but maternal vital signs do not change for blood loss of 1500 cc,
so vital signs cannot be trusted as an indicator of blood loss. Also, serious
thromboembolic disease is more common
during pregnancy.
E. Gastrointestinal System
1. Decreased motility, probably due to influence of
progesterone
2. Reduced gastric add secretion
Pertinence: A pregnant woman is
considered to have a full stomach even if she has had nothing to eat or drink
for several hours. Peptic ulceration is
rare during pregnancy.
F. Reproductive System
1. The Uterus (see figure 4)
a. Weight:
increases from 70 gm to 1100 gm
b. Blood
flow: increases to about 750 cc/min, or about 10-15% of cardiac output
Pertinence: Laceration of the uterine
arteries can result in massive hemorrhage in a short period of time.
2. The Cervix
a. increase in water content and vascularity
(Hegar's sign)
b. increase in cervical mucous secretions
III. Nutritional Considerations in the Normal Pregnancy
A. Weight gain: both weight gain and pre-pregnancy
weight are directly related to infant birthweight
1. Average weight gain (no one knows optimal weight
gain)
a. Normal weight for height: about 20 lbs
b. Underweight women: about 30 lbs
c. Overweight women: about 16 lbs
2. Average weight gain by organ system
a. Fetus-7 1/2 Ibs
b. Placenta and amniotic fluid-3 lbs
c. Blood volume-4 lbs
d. Breasts-1 to 2 lbs
e. Maternal fat--4 lbs
B. Daily dietary requirements for common nutrients
1. Calories: increased 15% kcal/day, or you need
about 2200 cal/day
2. Protein: an additional 10 to 30 gm /day (about 75
gm/day total)
3. Iron: supplement 30 to 60 mg of elemental iron
per day
4. Calcium: 1200 mg needed per day, usually provided
by a quart of milk per day (can use 2 Tums per day, each have 600 mg of calcium
carbonate)
5. Folate: supplement 200 to 400 mcper day (most
vitamins have 1 mg)
a. In women with a prior history of having a baby
with a neural tube defect, supplementing with 4 mg per day has been shown to
decrease the risk of a recurrence in the next pregnancy
C. The pregnant patient is best served by having a
healthy balanced diet with iron and folate supplementation. Only rarely are
other vitamin supplements needed.
IV. Prenatal Care for the Normal Pregnancy
A. The first visit-The basic
decision: normal vs. high-risk
1. History
a. Menstrual history:
confirm the pregnancy
(1) Regularity, interval,
duration
(2) Last normal menstrual
period (LMP): characteristics and bleeding since then
(3) Assign an estimated date
of delivery (EDD): it is inappropriate for a patient to be past 20 weeks of
pregnancy without a definite EDD
b. Past obstetric history
(if any): for many conditions, if the patient had an abnormality in the first
pregnancy, then she is predisposed to a recurrence in subsequent pregnancies
(1) Length of gestation
(2) Birth weight: low
(IUGR/SGA) vs. high (LGA/macrosomia)
(3) Fetal/neonatal outcome:
alive vs. dead, impairments
(4) Length of labor
(5) Type of delivery:
vaginal vs. cesarean, breech vs. cephalic
(6) Other complications
(7) Type of anesthesia used
c. Past medical history
(1) Significant past
illnesses
(2) Permanent conditions:
hypertension, diabetes, seizure disorder, thyroid disease, and so on
(3) Previous surgeries: C/S,
gynecologic /abdominal surgery
(4) Medications: prolonged
therapy
d. Family history
(1) Look for conditions with
familial predilection: hypertension, diabetes, cardiac disease, genetic
abnormalities
e. Social history
(1) alcohol use, smoking,
drug abuse
(2) 8-9% of pregnant women
in the Salt Lake Valley have a positive urine for at least one drug of abuse
(3) occupational hazards
f. Genetic screening:
evaluate from patient and family history the risk forgenetic abnormalities (is
it above the usual 2-3% of all pregnancies)
(1) Risk of chromosomal
abnormalities increases with maternal age:
Age 35 1/204
Age 38 1/103
Age 40 1/65
Age 42 1/40
Age 44 1/25
2. Physical examination
a. Vital signs: are they
normal or not
b. General physical
examination: are there any concurrent undiagnosed me conditions
c. Abdominal exam: scars,
enlarged uterus, other masses
d. Pelvic examination:
uterine size (confirm dates), cervical examination, Pap smear, clinical
pelvimetry
(1) uterine size large for
dates: think twins or incorrect dates
(2) uterine size small for
dates: think IUGR or incorrect dates
(3) the next step:
ultrasound evaluation of the pregnancy
3.Laboratory data
a. CBC: make certain the
patient is normal for pregnancy
b. Serology for syphilis:
RPR, VDRL, confirm with FTA
c. Blood type, Rh, and
indirect Coomb's test: evaluate for blood group isoimmunization
d. Rubella titer
e. Hepatitis B screen. HIV
screen offered
f. Maternal serum
alpha-fetoprotein (MSAFP) at 15-18 weeks
(1) If elevated, then the
patient should be evaluated with a targeted ultrasound for fetal anomalies, including
neural tube defects and abdominal wall defects (gastroschisis and omphalocele)
(2) If low, then the patient
should be offered a genetic amniocentesis to evaluate the fetus for trisomy 21
(Down's syndrome)
g. Urinalysis and urine
culture
h. Pap smear: abnormal
smears must be evaluated during pregnancy
i. Bacterial vaginosis (BV)
screening: wet mount
B. Subsequent visits
1. Frequency: the usual
regimen
a. Monthly up to 32 weeks
b. Every two week until 36
weeks
c. Weekly after 36 weeks
until delivery
2. Interval history
a. General health and
well-being
b. Presence or absence of
contractions
c. Fetal movement increased,
decreased
d. Leaking dear fluid: rule
out spontaneous rupture of membranes
e. Vaginal bleeding: all
vaginal bleeding after the first trimester is abnormal and mandates an
evaluation
3. Examination
a. Maternal weight
b. Blood pressure: get
worried if it is much above 120/80
c. Fundal height, estimated
fetal weight, fetal position (see figure 5)
d. Always confirm the
presence of fetal heart tones (FHT's)
e. Urinalysis for protein
and glucose: simple inexpensive screens for pre-eclampsia and diabetes
4. Laboratory evaluation
a. CBC in the early third
trimester: rule out anemia
b. Glucola (diabetes screen)
in the late second trimester
c. Rhogam at 26-28 weeks if
the patient is Rh negative
5. Ultrasound evaluation:
routine vs. indicated?
6. Preparation for labor
a. Childbirth education
classes
b. Physician input
C. Some common complaints
during pregnancy: the "Discomforts of Pregnancy"
1. Nausea and vomiting:
usually dissipates by 15 weeks or so
2. Constipation: common
throughout pregnancy
3. Heartburn: often worsens
as pregnancy progresses
4. Vaginitis: treat only if
symptomatic
5. Varicose veins and
hemorrhoids: treat symptomatically
6. Headaches
7. Edema: lower extremity
edema is very common
8. Nasal congestion and
nosebleeds,
9. Backache: lordosis is
common with change in the center of gravity
10. Leg cramps: especially
in lower leg
11. Faintness and
light-headedness
12. Breast tenderness
13. Carpal tunnel syndrome
D. Common questions for
which you will need to have an answer
1. Activity and exercise:
moderation should be encouraged
2. Sexual activity: no
problem as long as pregnancy progresses normally
3. Diet: a general balanced
diet is usually all that is required
4. Bathingng and swimming:
no high speed sports or jet skis
5. Douching: OK if pregnancy
is normal, best avoided if possible
6. Dentition: a dental
check-up is recommended, any work is OK
7. Immunizations: should
probably avoid live virus vaccines
8. Travel: no problems, but
should have frequent stops to stretch
9. Employment usually no
contraindication as long as pregnancy is normal
V. Take-home Points
1. The pregnant patient is
best served by a confident and caring physician who vigilantly searches for
high-risk features and then treats the patient as is appropriate for each
high-risk condition. Most patients have completely normal pregnancies, but the
high-risk pregnancy mandates changes in the "normal" evaluation of the
pregnant patient.
2. The process of antenatal
care is on-going risk assessment:
a. What is the genetic risk?
(maternal age, abnormal MSAFP screening, folate administration for prior NTD)
b. What is the risk for
preterm birth? (BV screening, history of preterm birth)
c. What is the risk for
pregnancy-induced hypertension? (history)
d. What is the risk for
IUGR? (past history, small uterine size for dates)
e. What is the risk for
blood group isoimmunization? (Rhogam for Rh- women NOT previously sensitized)


PHYSIOLOGY OF NORMAL LABOR AND DELIVERY - PART I
AND II
Objectives
1. To understand and recognize a normal labor pattern.
2. To understand the mechanism of labor for a cephalic
presentation.
3. To understand the meaning of the following germs: Presentation,
position, lie, station, effacement, dilatation.
4. To understand the phases and stages of labor.
5. To understand the following abnormalities of labor:
Prolonged latent phase, arrest of dilatation, and arrest of descent.
6. To understand the indications for cesarean delivery.
7. To understand the indications for forceps delivery.
Definitions
Attitude: This refers to the posturing of the joints and relation of fetal parts
to one another. The normal fetal attitude when labor begins is with all joints in
flexion.
Lie:
This refers to the longitudinal axis of the fetus in relation to the mother's
longitudinal axis (i.e., transverse, oblique, or longitudinal (parallel).
Presentation: This describes the part on the fetus lying over the inlet of the pelvic
or at the cervical os.
Point of Reference of Direction: This is an arbitrary point on the presenting
part used to orient it to the maternal pelvis [usually occiput, mentum (chin)
or sacrum].
Position: This describes the relation of the point of reference to one of the
eight octanes of the pelvic inlet (e.g., LOT: the occiput is transverse and to
the left).
Engagement: This occurs when the biparietal diameter is at or below the inlet of
the true pelvis.
Station: This references the presenting part to the level of the ischial spines
measured in plus or minus centimeters.
Flexion and Engagement: This occurs at various times before the forces of
labor begin.
Descent: This occurs as a result of active forces of labor.
Internal Rotation: This occurs as a result of impingement of the presenting part on the
bony and soft tissues of the pelvis.
Extension: This is the mechanism by which the head normally negotiates the pelvic
curve.
External Rotation (Restitution): This is the spontaneous realignment of the
head with the shoulders.
Expulsion: This is anterior and then posterior shoulders, followed by trunk and
lower extremities in rapid succession.
I. The Characteristics of
Uterine Contraction in Labor
The musculature of the
pregnant uterus is arranged in three strata:
1. An external hood-like layer which arches over the fundus and
extends into the various ligaments.
2. An internal layer consisting of sphincter-like fibers around
the orifices of the tubes and internal os.
3. Lying between the two, a dense network of muscle fibers
perforated in all directions by blood vessels. The main portion of the uterine
wall is formed by this middle layer which consists of an interlacing network of
muscle fibers between which extend the blood vessels. As the result of such an
arrangement, when the cells contract after delivery, they constrict the vessels
and thus act as "living ligatures."
Uterine contractions are
involuntary and, for the most part, independent of extrauterine control. It has
been demonstrated that the uterus has pacemakers to produce the rhythmic
coordinated contractions of labor. These pacemaker sites are found near the
uterotubal junctions, although the pacemaker cells do not differ anatomically
from the surrounding myocytes as they do in cardiac muscle. The interval
between contractions diminishes gradually from approximately ten minutes in
early labor to as little as two minutes near the end of labor. In the normal
process there is a progressive increment in the strength of contractions from
approximately 20 mm of mercury at the onset of labor to 50 to 80 mm late in
labor. The effect of uterine contractions of this frequency and intensity is
twofold on the uterine cervix. First effacement consisting of thinning of the
cervix with a shortening of the endocervical canal, is produced. Secondly,
cervical dilatation concurs, initially slowly as it accompanies the process of
effacement of the cervix, and then more rapidly as cervical effacement has been
accomplished (see Figure 1).
Progressive contractile
activity of the uterus has been demonstrated throughout pregnancy. Most of
these contractions are 'imperceptible to the pregnant individual, but toward
the end of pregnancy they may achieve on a sporadic basis strength equivalent
to those of early labor. False labor, Braxton-Hicks contractions, and pre-labor
contractions are terms that have been applied to this uterine activity. The
latter term is probably the most appropriate, and it is this uterine activity,
which accomplishes a significant degree of effacement and even some dilatation
in the days or weeks prior to the onset of recognizable labor. Descent of the
presenting part of the fetus into the birth canal, particularly in a first
pregnancy, is another result of pre-labor.
II. The Mechanism of Normal Labor
The definition or clinical
diagnosis of labor is a retrospective one. There is no laboratory test that
gives a "labor titer" or an x-ray procedure that can define the
difference between the laboring and non-laboring patient. Realizing these
limitations, the patient is diagnosed as being in labor when a combination of
conditions exists. Perhaps a good working definition may be stated as follows:
When in the presence of perceived uterine contractions, there is progressive cervical dilatation and
descent of the presenting part which leads to the eventual expulsion of the
products of conception, the patient is in labor.
The "mechanism of
labor" refers to the sequencing of events related to posturing and
positioning that allows the baby to find the "easiest way out." For
the most part the fetus is a passive respondent in the process of labor, while
the mother provides the uterine forces and a structural configuration of the
passageway through which the passenger must travel. For a normal mechanism of
labor to occur, both the fetal and maternal factors must be harmonious. An
understanding of these factors is essential for the obstetrician to
appropriately intervene if the mechanism deviates from the normal. The
definitions at the beginning of this section should mastered to be able to
discuss and understand the mechanism of labor.
The single most important
determinant to the mechanism of labor is probably pelvic configuration. The
classic work of Caldwell and Maloy is reviewed in the text and should be
understood. Their classification of the pelvis into four major types (gynecoid,
android, anthropoid, and platypelloid) helps the student understand the
possible difficulties that may arise in a laboring patient. A quote that should
be remembered is: "No two pelves are exactly the same, just as no two
faces are the same. For each pelvis there is an optimum mechanism that may be
wholly different from the so-called normal mechanism described."
An important principle is
that most pelves are not purely defined but occur in nature as mixed types.
Regardless of the shape, the baby will be delivered if size and positioning
remain compatible. The narrowest part of the fetus attempts to align itself
with the narrowest pelvic dimensions (e.g., biparietal to interspinous
diameters) which means the occiput generally tends to rotate to the "most
ample portion of the pelvis."
The mechanical steps the
baby undergoes can be arbitrarily divided, and clinically they are usually
broken down into six or eight steps for ease of discussion. It must be
understood, however, that these are arbitrary distinctions in a natural
continuum.
The following six divisions
of labor are easy to use:
1. Flexion and Engagement. This occurs
at various times before the forces of labor begin.
2. Descent. This occurs
as a result of active forces of labor.
3. Internal Rotation.
This occurs as a result of impingement of the presenting part on the bony and
soft tissues of the pelvis.
4. Extension. This is the mechanism by
which the head normally negotiates the pelvic curve.
5. External Rotation
(Restitution). This is the spontaneous realignment of the head with the
shoulders.
6. Expulsion. This is anterior and then
posterior shoulders, followed by trunk and lower extremities in rapid
succession.
Abnormal mechanisms of labor
do occur, and the operator must be able to recognize these early and intervene
when appropriate. Those patients who
have undeliverable or uncorrectable problems should be unhesitatingly delivered
by the abdominal route because inappropriate operative vaginal intervention may
lead to damage to both mother and fetus. Some of the undeliverable situations
include persistent mentum posterior, persistent brow presentation, some types
of breech presentations, and shoulder presentation.
Principle movements in the mechanism of labor and delivery

III.
Physiology of Normal Labor and Delivery
A. Normal labor
Emanuel Friedman in his
elegant treatise on labor (1978) stated correctly that the clinical features
of uterine contractions namely frequency, intensity, and duration cannot be
relied upon as measures of progression in labor nor as indices of normality.
Except for cervical dilatation and fetal descent, none of the clinical features
of the parturient patient appears to be useful in assessing labor progression.
Friedman sought to select criteria that would limit normal labor and thus be
able to identify significant abnormalities of labor. These limits, admittedly
arbitrary, appear to be logical and clinically useful. The graphic
representation of labor plotting descent and dilatation against time has become
known as the Friedman curve. It, or a modification of it, is used extensively
to evaluate laboring patients.

Friedman Curve
Figure 2. Graphic portrayal
of the relationship between cervical dilatation and elapsed time in labor
(heavy line) and between fetal station and time (light line). Labor has been
divided functionally into a preparatory division (including latent and
acceleration phases of the dilatation curve), a dilatational division comprising
only the linear phase of maximum slope of dilatation, and a pelvic division
encompassing the linear phase of maximum descent.
B.
Functional
classification of labor
Principal Clinical Features on the
Functional Divisions of Labor
|
Characteristic |
Preparatory Division |
Dilatational Division |
Pelvic Division |
|
Functions |
Contractions coordinated,
polarized, oriented, cervix prepared |
Cervix actively dilated |
Pelvis negotiated;
mechanisms of labor; fetal descent delivery |
|
Interval |
Latent and acceleration
phases |
Phase of maximum slope |
Deceleration phase and
second stage |
|
Measurement |
Elapsed duration |
Linear rate of dilatation |
Linear rate of descent |
|
Diagnosable disorders |
Prolonged latent phase |
Protracted dilatation;
protracted descent |
Prolonged deceler-ation;
secondary arrest of dilatation; arrest of descent; failure of descent |
C. Abnormal labor
Dystocia (literally difficult labor) is characterized by abnormally slow progress in labor. It is the consequence of four distinct abnormalities that may exist singly or in combination.
1. Uterine forces that are not sufficiently strong or appropriately coordinated to efface and dilate the cervix.
2.
Forces
generated by voluntary muscles during the second stage of labor that are
inadequate to overcome the normal resistance of the bony birth canal and
maternal soft parts.
3.
Faulty
presentation or abnormal development of the fetus of such character that the
fetus cannot be extruded through the birth canal.
4.
Abnormalities
of the birth canal that form an obstacle to the descent of the fetus.
Labor Disorders
|
Pattern Pattern |
Diagnositc Criterion |
|
Prolonged latent phase |
Nulliparas 20 hr or more Multiparas 14 hr or more |
|
Protracted active phase
dilatation |
Nulliparas 1.2 cm/hr or
less Multiparas 1.5 cm/hr or
less |
|
Protracted descent |
Nulliparas 1 cm/hr or less Multiparas 2 cm/hr or less |
|
Prolonged deceleration
phase |
Nulliparas 3 hr or more Multiparas 1 hr or more |
|
Secondary arrest of
dilatation |
Arrest 2 hr or more |
|
Arrest of descent |
Arrest 1 hr or more |
|
Failure of descent |
No descent in decleration
or second stage |
1.
Prolonged
latent phase of labor

Arrest
disorder

A - Secondary anrst of dilatation pattern with documented cessation of
progression in the active phase
B - Prolonged deceleration phase pattern with deceleration phase
duration greater than normal limits
C - Failure of descent in the deceleration phase and second stage
D - Arrest of descent characterized by halted advancement of fetal
station in the second stage.
These four abnormalities are similar in etiology, response to
treatment, and prognosis, being readily differentiated from the normal
dilatation and descent curves (broken lines).
 Etiology of arrest disorders
are: Power (inadequate uterine
contractions), Passage (cephalo-pelvic disproportion pelvis too small or
inadequately shaped for delivery), and Passenger (fetus too large or presenting
abnormally).
Etiology of arrest disorders
are: Power (inadequate uterine
contractions), Passage (cephalo-pelvic disproportion pelvis too small or
inadequately shaped for delivery), and Passenger (fetus too large or presenting
abnormally).
IV. Forceps
delivery

Forceps
Figure 5. Showing line of
axis traction perpendicular to the plane of the pelvis at which the head is
stationed.

V. Cesarean delivery
PIH/OBSTETRICIAL HEMORRAGE
WORKSHOP
I. Preeclampsia and Eclampsia
Case Presentation A 22-year-old Primigravid patient at 32
weeks of gestation presents with a blood pressure of 140/96, a urialysis
showing 2 + protein, and a 5 lb (2.27 kg) weight gain in two weeks. Her cervix
is 1 cm dilated, uneffaced with a floating cephalic presentation. She is admitted to the hospital for a
nonstress test (NST) which is reactive.
Her laboratory values are within normal limits. She is observed at be rest, and over the
course of the next 24 hours her blood pressure increases to 150 to 160/100 to
110. She develops 3+ proteinuria and
complains of epigastric pain.
Terminal Objective Given a patient with hypertension, the
student should be able to make an appropriate diagnosis and establish a plan of
management.
Enabling Objectives The student should be
able to:
1.
Define
the various hypertensive disorders in pregnancy and the underlying
pathophysiology.
2.
Know
the incidence, clinical course, prognosis, prophylaxis and general management
including pharmacologic agents used for these disorders.
II. Late
Pregnancy Bleeding
Case Presentation - A 32-year-old, gravida 6, para 5-0-0-5 at
28 weeks of gestation presents with vaginal bleeding to the emergency
room. Her obstetrician is out of
town. Her vital signs show a blood
pressure of 100/50, a pulse of 98, and respiratory rate of 24. She is extremely anxious. Her obstetrical history and current prenatal
course is unremarkable.
Terminal Objective Given a pregnant patient in the third
trimester who is bleeding per vagina, the student should be able to evaluate
the case and discuss the management.
Enabling Objectives The student should know.
1.
The
most common obstetrical and non-obstetrical causes and overall incidence of
bleeding late pregnancy.
2.
If
the unsuspected diagnosis is abruptio placentae, know the pathophysiology,
clinical characteristics, maternal and fetal complications and management.
3.
If
the suspected diagnosis is placenta previa, know the classification, incidence
and probable mechanism, methods to localize the placenta clinical
characteristics and management
Preeclampsia and Eclampsia
I. Definition
Preeclampsia is that condition occurring only during pregnancy characterized by
hypertension, edema and proteinuria.
Eclampsia is the occurrence of convulsions, not caused by any coincident
neurologic disease in a woman whose condition fulfills the criteria for
preeclampsia. The etiology of preeclampsia
is unknown. This condition occurs only
in humans, and there is no animal model.
Preeclampsia, can occur any time after 20 weeks of gestation but usually
becomes clinically evident late in pregnancy.
It occurs in 7% of all pregnancies.
Another term commonly seen is pregnancy-induced hypertension (PIH) which
is divided into three categories, (1) Hypertension alone, (2) preeclampsia, and
(3) eclampsia
PIH is classified as mild or severe according to the
following:
|
Abnormality |
Mild |
Severe |
|
Diastolic
BP |
<100
mm HG or rise >15
mm Hg from second
trimester BP |
110
mm Hg or greater or rise >30 mm Hg from second trimester BP |
|
Proteinuria |
Trace
to 1+ |
Persistent
2+ or more* |
|
Headaches |
Absent |
Present |
|
Visual
Disturbances |
Absent |
Present |
|
Epigastric
pain |
Absent |
Present |
|
Oliguria |
Absent |
Present |
|
Convulsions |
Absent |
Present |
|
Serum
creatinine |
Normal |
Elevated |
|
Thrombocytopenia |
Absent |
Present |
|
Hyperbilirubinemia |
Absent |
Present |
|
SGOT
elevation |
Minimal |
Marked |
|
Fetal
growth retardation |
Absent |
Obvious |
|
Pulmonary
edema |
Absent |
Present |
*Significant proteinuria is defined as >
300mg/24 hour collection or 100 mg/dl on two separate urine collections >
six hours apart (2+ on a urine dipstick)
II. Incidence-Occurs in 6 to 8% of pregnancies and continues to
be one of the leading causes of Maternal morbidity and mortality. It ranks as the second leading cause of
maternal death after pulmonary embolism.
III. Risk Factors
1.
First
pregnancy 85% of cases of PE/E occur in first pregnancies.
2.
Age-PE/E
occurs most frequently in teens and women in their late 30s and 40s.
3.
Chronic
hypertension
4.
Renal
disease
5.
Molar
pregnancy
6.
Previous
pregnancy complicated by severe PE/E
IV. Management- While PE does not usually become clinically evident until late in pregnancy it is felt to develop a the time of implantation and is, therefore, directed toward preventing severe complications for the mother or baby. Low-dose aspirin has recently become popular in physician attempts to prevent the occurrence of PE. Conclusive evidence may be forthcoming.
CONDITION THERAPY
A. PIH
with mature fetus 1. Prevent convulsions
2. Control BP
3. Delivery
B. PIH with immature fetus bet there is:
1.
Severe
PE or 1. Prevent convulsions
2.
Fetal
growth retardation or
2. Control BP
3.
Fetal
jeopardy 3. Delivery
C. PIH
with immature fetus and PIH Expectant
management
Is
mild or moderate
D. Patients at risk for PIH Consider low-dose aspirin (15 mg/day)
The indication for
hospitalization of women with PE is a systolic blood pressure
of 140 mm. Hg and greater or a diastolic blood pressure of 90 mm Hg and greater.
Hospital Management:
1.
History
and physical exam followed by daily search for evidence of development of
severe PIH
2.
Weight
measured every two days.
3.
Urine
check for proteinuria every two days (24-hour collections)
4.
BP
check every four hours except at night to allow sleep.
5.
Follow
labs CBC, Platelets, SGOT, Creatinine
6.
Monitor
fetal well-being and growth.
Drug therapy to prevent convulsions and
control BP
1.
Magnesium
sulfate-typically administered intravenously, monitoring reflexes, respirations
and urine output.
2.
Hydralazine
(Apresoline) used to control sever HTN and reduce the maternal risk of
intracranial hemorrhage, NOT to achieve normotension which can compromise
placental perfusion. Several other
antihypertensive drugs can be used in this setting (beta blockers). However, the bulk of experience is with
hydralazine.
Delivery is achieved by induction of labor and use of ceasarean section for fetal or obstetrical indications.
V. Complications-Most serious complications of PE, including
eclampsia, DIC, ruptured liver, maternal stroke, and fetal death, are due to
failure to recognize the severity of the disease, failure to hospitalize the
patient or failure to move to delivery.
The goal of management of this disease is to keep the mother healthy and
prevent intrauterine fetal demise. The
earlier the onset of the illness, the more severe the course with regard to
mother and fetus. The overall neonatal
mortality rate in 1985 in the USA was 7/1000 live births. When toxemia is not managed, the neonatal
death rate can increase 10 to 15 times.
Third
Trimester Bleeding
1. Placenta Previa
A. Definition-Place:
Implantation in the lower uterine segment over or near the cervical
internal os. Placenta previa is uncommon with the first child. In women who have had a previous cesarean
section and present with an anterior low-lying placenta previa, up to 25% of
these women may have a placenta acreta caused by the absence of Nitabuchs
layer. This can result in not only a
cesarean section but also a postpartum hysterectomy commonly referred to as a
cesarean hysterectomy.
Incidence-approximately 1 in 200 pregnancies.
Types
1.
Total
placenta previa-cervical os completely covered by placenta.
2.
Partial
placenta previa-cervical os partially covered by placenta.
3.
Marginal
placenta, previa-placental edge is at the margin of the cervical os.
4.
Low-lying
Placenta-placental implantation in the lower uterine segment, but the placental
edge does not actually reach the internal os.
B. Signs and Symptoms
1.
Painless
hemorrhage- usually occurring toward the end of the second trimester or later
2.
Coagulation
defects are rare.
C. Risk Factors
1.
Multipara
2.
Advanced
maternal age
3.
Previous
cesarean section
4.
Large
placenta such as in multiple gestation and pregnancies complicated by fetal
erythroblastosis.
5.
Previous
D&C or treatment for Ashermans syndrome
D. Diagnosis:
The diagnosis is suspected in the setting of painless bleeding late in pregnancy, a uterus which is soft and nontender, and a presenting part which is high in the uterus. The diagnosis in confirmed by ultrasound. It is dangerous to do a vaginal examination on a patient with third trimester bleeding since this may precipitate uncontrollable hemorrhage. Blood flow to the uterus at term ranges from 400 to 800 cc/minute.
E. Management
In the setting of a premature fetus and no active bleeding, hospitalization and close observation is standard. Delivery by cesarean section is indicated in the setting of fetal maturity or severe maternal hemorrhage.
2. Placental Abruption
A. Definition
The separation of the placenta from its site of implantation in the uterus before the delivery of the baby.
B. Signs and Symptoms
Very variable, bleeding may be heavy or nonexistent. Pain may be severe or mild, and the baby may die or be unaffected. Placental abruption can be complicated by severe maternal hemorrhage, coagulation defects, renal failure, and fetal demise.
C. Risk Factors
1.
Trauma
2.
Polyhydramnios
3.
Chronic
HTN or PIH
4.
Short
umbilical cord
5.
Uterine
anomaly or tumor
6.
Alcohol
or drug use (particularly cocaine)
D. Diagnosis
The diagnosis is made by clinical suspicion. Ultrasound only occasionally confirms the diagnosis.
E. Management
Treatment will vary depending on the well-being of the mother and baby. Cesarean section is indicated for fetal distress and maternal hemorrhage. Close monitoring of fluid balance and coagulation defects is essential to optimize outcome.
III. Other Causes
A. Marginal sinus rupture
B. Uterine rupture
C. Bloody show of labor
D. Vasa
Previa
E. Non-obstetric
causes-vulvovaginal trauma, cervical lesions
F. Unknown
IV. Initial approach to a patient presenting with third-trimester bleeding
A. Hospitalization
B. Careful abdominal examination, including Leopold maneuvers
C. No internal vaginal or rectal exams
D. Placement of IV access
E. Type and crossmatch 2 to 4 units of PRBC
F. Ultrasound examinations for placental location
G. Close monitoring of mother and baby
MALE REPRODUCTION
Douglas
Carrell, Ph.D.
Objectives
1. To understand hypothalamic-pituitary-testicular hormonal
axis and the role of hormones in spermatogenesis.
2. To understand the process of sperm production.
3. To know the role of and the markers for epididymis, vas
deferens, seminal vesicles, and the prostate.
4. To understand male fertility problems including: (a)
diagnosis of male infertility, (b) traditional treatment of male infertility,
and (c) advanced reproductive techniques.
Definitions
Aspermia: The failure to produce an
ejaculate.
Asthenospermia
(azthenozoospermia): The production of an ejaculate in which less than 50% of spermatozoa
are motile.
Azoospermia: The production of an
ejaculate devoid of spermatozoa.
Oligospermia
(oligozoospermia): The production of an ejaculate containing less than 20 million
spermatozoa per milliliter of semen.
Teratospermia
(teratozoospermia) - The production of an ejaculate in which more than 50% of spermatozoa
are of abnormal shape.
Hormones and
Male Reproduction
I. Hypothalamus
A.
GnRH
(LHRH) contributes to the release of both LH and FSH from the pituitary.
III Pituitary
A. FSH
release is controlled by the feedback of inhibin from the testicle.
B. LH
release is controlled by the feedback of steroids from the testicle.
C. LH
and FSH also control their own release by feeding back to hypothalamus.
D. The
target organs for FSH and LH are:
1. LH acts on the Leydig
cells to increase steroidogenesis.
2. FSH
participates in protein synthesis and the initiation of spermatogenesis at the
level of the seminiferous tubules.
III. Testicle
A. LH binds
to Leydig cells and increases cAMP which increases protein secretion and the
side-chain cleavage of cholesterol, as well as other likely steps, to increase
steroidogenesis and the production of testosterone and other androgens.
Regulated by steroid feedback. The Leydig cells produce the testicular
steroids, lie between the seminiferous tubules, and assist in the
transportation of steroids in the blood, lymph and seminiferous tubules.
B.
FSH
binds to the Sertoli cells of the seminiferous tubules, increases cAMP and
protein synthesis, androgen binding in the tubules, etc. Regulated by inhibin
produced by the Sertoli cells. Sertoli cells secrete proteins that are
important to spermatogenesis and have been called the "director cells of
spermatogenesis." They comprise the blood-testis barrier.
C.
Prolactin
may increase Leydig cell response to LH and/or prostate sensitivity to
androgens.
D.
Steroids
and other hormones may aid in the movement of sperm from the testicle by
causing smooth muscle contractions.
E.
Normal
hormone values:
FSH = 1-10 mIU/ml
LH = I -10 mIU/mI
Testosterone = 3-10 ng/mI
Estradiol
and dihydrotestosterone = extremely low in normal males.
F. Spermatogenesis occurs with the following steps:
1. Yolk
sac endoderm. gives rise to primordial germ cells which give rise to more type
A cells, some of which degenerate.
2. Type A
stem cells form additional type A cells or differentiate into type B
spermatogonia cells during early puberty.
3. Type B
cells differentiate during late puberty and in the adult to form primary
spermatocytes, secondary spermatocytes and spermatids. These events occur
initially through mitosis and then reduce the chromosomes to one-half through
miosis.
4. Spermiogenesis
transforms early spermatids into late spermatids and form what we recognize as
morphological normal sperm.
5. The above process takes
72 to 74 days in the human.
6. Sperm
are released into the lumen through spermiogenesis which involves a gradual
7. Disorders of
spermatogenesis can occur and can include:
a. Azoospermia
including Sertoli cell only syndrome
b. Maturation
arrest at one of a number of possible stages
c. Hypospermatogenesis
8. Sperm
transport from the testicle occurs through:
a. Seminiferous tubule contractions of the myoid cells (hormone
dependent)
b. Fluid
build up and pressure
c. Testicular
capsule contractions
IV. Epididymis
A. Responsible for sperm
maturation and motility.
B. Usually two-thirds of
the epididymis is needed for sperm transport in the human for normal sperm
fertilization capacity.
C. Potential markers of
epididymal function include carnitine and glycerol phosphocholine.
D. Sperm take approximately two weeks to
get through the epididymis.
E. Sperm are stored
near the tail portion and in the vas deferens until they are ejaculated.
V. Accessory organs
A. Seminal
vesicles
1.
Contribute prostaglandins and fructose.
2. Contribute two-thirds of volume of ejaculate.
3. Qualitative
fructose is useful for verifying presence and the presence of the vas deferens.
B.
Prostate
1.
Contributes acid phosphatase, Zn and citric acid
2.
Chronic inflammation may contribute to infert
3. Many markers including PSA
C. Vas
deferens
1. Sperm storage and
transport organ
2. Adrenergic innervation
VI. Male infertility
A.
Contributes to about 50% of infertility.
B. Many
potential causes including:
Varicocele
Idiopathic
Testicular failure
Obstruction
Cryptorchid
Volume
Agglutination
Sexual dysfunction
Viscosity
Ejaculatory failure
Endocrine
High density
Necrospermia
Combination of sperm defects
C. Varicocele contributes to
about 40 %; idiopathic causes are about 25%
D. Diagnosis
1. Physical exam
a. Testicular size and
consistency
b. Varicocele
- usually on the left side; on both sides or right about 10% of the time.
2. Semen quality
a. Two or
more complete semen analyses
b. Should
evaluate volume, agglutination, viscosity, quantitative motility and
morphology, count, viability and membrane quality.
3. Blood hormones
a.
FSH
b.
LH
C.
Testosterone
d. Prolactin
4. History
E. Treatment of male
infertility
1. Specific medical problems
a.
Gonadotropins - HCG, Pergonal, Metrodin
b.
Other - thyroid, adrenal, etc.
2. Non-specific medical therapy
a. Gonadotropins - 2500 IU HCG 2X weekly - 10 to 12 weeks
b. Testosterone rebound therapy
c. Clomiphene citrate - 25 mg daily for 3 to 4 months
3. Varicocele Semen pattern of low motility, high tapered and
immature sperm
Post-surgery correction yields pregnancy rates of
30% to 60% and semen improvement rates of 60% to 80%.
4. Laboratory improvement of sperm with insemination
a. Can make semen quality better with lab manipulation
techniques and then utilize with intrauterine insemination.
5. Laboratory improvement with advanced reproductive techniques
a. GIFT techniques
b. IVF techniques
(1) Regular IVF
(2) ICSI techniques for poor sperm quality
(3) Sperm aspiration or testicular biopsy with ICSI
Take-Home Points
The history, physical exam,
and laboratory investigations will detect an etiology for male factor
infertility in approximately 50% of cases. Serum FSH levels provide an
important diagnostic parameter in determining the pathological basis of
azoospermia. Karyotypic: analysis is most helpful in males with azoospermia and
small testes. Obstruction of the ejaculatory ducts can be diagnosed by
ultrasound. The absence of the vas deferens can be detected by the absence of
fructose in the semen sample.
The
most common congenital abnormality resulting in testicular dysfunction is
cryptorchidism. The longer the testis remain outside the scrotum, the greater
the degree of spermatic disruption. The most common chromosomal abnormality
resulting in deficient testicular function is Klinefelter's syndrome. The
frequency of this abnormality is 1 in 500 live births. The 47,XX7 karyotype
results in the destruction of all germ cells with seminiferous tubules causing
small, firm testes and azoospermia. Gynecomastia and various degrees of
androgen deficiency are usually noted. The most common vascular abnormality
associated with infertility is a varicocele. The higher frequency of varicocele
in infertile men (21 % to 41 %) compared to men in the general population (4 %
to 23 %) has been interpreted as supporting a causal relationship between
varicocele and infertility. Theories to account for adverse testicular function
with a varicocele include: vascular stasis, back pressure, interference with
oxygenation, reflux of renal or adrenal products into the pampiniform plexus
and interference with heat exchange function of the pampiniform plexus.
GYNECOLOGIC ENDOCRINOLOGY SEMINAR
INFERTILITY
GOALS OF THE ASSESSMENT AND
TREATMENT : Objectives
a) Establish a diagnosis
efficiently
b) Educate the infertile
couple
c) Establish a prognosis
d) Establish and effect a
realistic plan
e) Emotional support for the
couple
f) Advise when
discontinuation of treatment should be considered
DEFINITIONS (see also
glossary on the last page)
Infertility: The
inability to conceive following 12 months of regular coitus without
contraception. In couples who conceive normally, 50% do so following 3 months
whereas about 92% conceive following 12 months.
Fecundity: The
monthly probability of pregnancy. (Normally 20-25 % at best)
Primary
infertility: Conception has never taken place.
Secondary infertility: At
least one previous conception has been documented.
Sterility: The etiology of
infertility is established and there is no possibility for conception.
DEMOGRAPHICS
Over the past two
decades, the number of patient visits for infertility have soared. This does
not appear to be caused by an increased proportion of infertility within age
groupings. Rather, factors contributing to the demand include: a higher
absolute number of couples of reproductive age ("baby boomers"),
decreasing availability of babies for adoption (largely due to legalized
abortion and increasing social acceptance of single parenthood), less stigma
regarding infertility, increasing tubal disease due to sexually transmitted
disease, and better and more plentiful medical care providers for infertility
services. However, the most important contributor to the increased prevalence
of infertility visits is delayed childbearing with consequent attrition of
ovarian function.
Approximately
10-15% of all married couples in the United States are infertile. A systematic
approach should be used to evaluate the cause(s) of infertility for a couple,
while the emotional stress that accompanies infertility for both partners must
be constantly addressed.
The
etiology of infertility can be divided into three major categories: (1) female
factor, (2) male factor, and (3) undetermined etiology. Approximately 40% of infertility
cases, where the etiology has been determined, are due to female factor, 40 %
to male factor, and the remaining 20% are due to mixed male/female factors. In
10-20% of couples presenting for evaluation, no diagnosis can be made after
standard investigation (unexplained infertility).
INVESTIGATION PLAN
Investigation of infertility should be designed to
be completed as efficiently as possible. All couples should have a complete history
and physical. The most information can be obtained if both partners are present
initially. A sexual history should be obtained, including the frequency and
timing of intercourse, the use of potentially spermicidal lubricants and a
complete menstrual history.
Female Infertility
Etiologies
Central (CNS)
Tubal
Pelvic/peritoneal
Endometrial/uterine
Cervical/mucus
Unexplained
The entire reproductive axis
(hypothalamus, pituitary, ovary, pelvis, fallopian tubes, uterus, and vagina)
must be intact and integrated for the success of the reproductive system.
Systematic consideration of major risk factors for each component should be
considered during history taking. Examples of some of the principal
dysfunctions for each component of the reproductive system are given:
Sperm- childhood mumps,
testicular injury/sexually transmitted diseases, varicocele, sexual
dysfunction, exposure to toxins, endocrinopathies
Hypothalamus- extremes of
weight, stress, excessive exercise, CNS lesions, Kallman's Syndrome, eating
disorders
Pituitary- tumors
(Prolactinoma, Cushing's Disease, Acromegaly), Postpartum panhypopituitarism
(Sheehan's Syndrome).
Ovaries- tumors, surgical
trauma, endometriosis, radiation / chemotherapy damage, dysgenetic gonads,
polycystic ovary syndrome
Gametes/ folliculogenesis-
age, smoking, medications. illicit drug / alcohol use (either partner),
exposure to radiation, toxic chemicals, premature ovarian failure, obesity
Pelvis/Fallopian Tubes- IUD
use, pelvic inflammatory disease, ruptured appendix, previous ectopic
pregnancy, prior pelvic surgery, endometriosis
Uterus- embryologic
malformations (e.g. septate uterus), luteal phase inadequacy, intrauterine
synechiae (Asherman's Syndrome), leiomyomata uteri
Cervix- history of
diethystilbesterol exposure, previous cervical surgery
Vagina- embryologic
malformations e.g. imperforate hymen
Systemic disease- diabetes,
renal failure, thyroid dysfunction, anorexia nervosa, etc.
REVIEW OF SECTION I
Be aware of the
most important factors in society that have contributed to infertility as major
health care concern.
Realize that each
anatomical component of the reproductive system must function appropriately for
a successful pregnancy; and the easiest way to take a careful history is to
consider each component systematically
II. PHYSICAL EXAMINATION AND LABORATORY INVESTIGATION
The goals of this
section are to learn to look for clues on physical examination to common
infertility causes. Also, the basic tests used to evaluate each component of
the reproductive system will be reviewed.
PHYSICAL EXAMINATION
As with history
taking, the physical examination is best directed toward uncovering
manifestations of pathology involving each individual component of the
reproductive system. In particular, attention is paid to the patient's weight,
thyroid palpation, evidence of acne, hirsutism and seborrhea, as well as a
search for galactorrhea. The degree of development of secondary sexual
characteristics is also noted. Pelvic exam offers many clues including
assessment of ovarian estrogen production (via observation of cervical mucus production
and vaginal cytology). Mullerian abnormalities, leiomyomata uteri, and other
pelvic masses and observation of any pelvic pain. A nonmobile retroverted
uterus may signify the presence of pelvic adhesions from previous PID,
surgeries, appendicitis or endometriosis that are fixing the uterus in the
pelvic cul-de-sac.
LABORATORY INVESTIGATION OF
INFERTILITY
Rubella
immunity status, chlamydia and gonorrhea cultures are generally pursued on the
initial visit. Folate supplementation should be considered if an inadequate
diet is ascertained (ingesting 0.4 mg of folic acid daily before conception,
decreases the probability of a neural tube defect by 50%). Then, an efficient
general approach is to begin with a semen analysis followed by presumptive
documentation of ovulation usually by temperature charting, and a
hysterosalpingogram (HSG). Attention must be paid to the time during the
menstrual cycle when the tests are performed since most have an optimal window
during which they should be done. Depending on the outcome of the
aforementioned tests, the following additional tests may be offered:
endometrial biopsies to establish the diagnosis of inadequate luteal phase and,
a postcoital test to determine sperm survival and movement within cervical
mucus, and lastly, diagnostic laparoscopy.)
a) Examination of Semen
Semen
analysis should be the first step in the investigation before expensive and
invasive female testing is performed. Semen specimens are best collected at the
andrology lab. If this is not possible,
specimens can be collected at home if they can be brought to the laboratory
within 30 minutes. The semen should be collected by masturbation in a clean,
detergent-free container. The interval of abstinence should be 48 to 72 hours.
Since most of the spermatozoa are found in the first milliliter of the
ejaculate, the man should be instructed to be careful to include this fraction.
At least
two specimens should be examined at least several weeks apart since there can
be considerable variability in quality. Like the female, the entire male
reproductive axis must be evaluated depending upon the particular sperm
abnormality. Abnormal results deserve referral to a urologist skilled in the
male infertility evaluation.
While the
individual parameters of the semen analysis are not particularly sensitive
predictors of fertility, the overall semen quality does have predictive value.
Minimum normal values have been suggested: sperm concentration > 20 million
per ml, total count > 60 million, ejaculate volume > 1.5 ml, total motile
count > 30 million, viable sperm > 50%, normal shapes (morphology) >
60%.
A host of specialized sperm
function tests are available when indicated, for example:
Antisperm antibody assays
Hamster egg penetration test
(HEPT)- to predict fertilizing capability of spermatozoa
Hypoosmotic swelling test
(HOS)- to assess sperm membrane function
b) Presumptive Documentation of Ovulation
The vast
majority (98%) of women who give a history of regular menstrual cycles combined
with premenstrual symptoms are probably ovulating. When in doubt,
several practical tests offer presumptive evidence of ovulation: BBT, serum
progesterone, and urinary LH surge testing. Endometrial biopsies and serial
ultrasound examinations to ascertain the collapse of ovarian follicles are
other ways to make the presumptive diagnosis of ovulation, but are less
practical and very expensive.
Basal Body Temperature
Under the
influence of circulating progesterone, the BBT rises in the luteal phase of the
cycle. A mean increase of at least 0.4*F over the proliferative-phase
temperature is considered normal. The patient should be instructed to take her
temperature each morning prior to getting out of bed. The BBT is free, and
answers the question of whether there is ovulation or not for the majority of
women. Also, the length of the luteal phase can be assessed. Drawbacks include
the fact that it can not predict prospectively when ovulation will occur, and
it can be bothersome to record. Digital electronic thermometers have made this
early morning task more palatable.
Serum Progesterone
A single serum progesterone
value above 4 ng/ml obtained between days 19 and 23 of the
menstrual cycle is
presumptive evidence of ovulation.
Urinary LH Surge Testing
Serum
estradiol is released from ovarian follicles in increasing amounts during the
follicular phase. When the serum estradiol concentration exceeds a threshold
level for a particular duration, positive feedback to the
hypothalamic-pituitary axis initiates the release of a surge of LH. This LH
surge triggers the events leading to ovulation approximately 40 hours after the
start of the surge. LH is excreted into the urine where is can be measured
simply by any number of commercially available ELISA home test kits. The major
advantage over BBTs and serum progesterone determinations is the ability to
prospectively predict the presence and timing of ovulation. LH kits are used in
our clinic twice daily to ascertain optimal timing of artificial inseminations.
When a
woman is found to be anovulatory, the underlying etiology must be sought and
corrected if possible. In brief, some of the more common causes of anovulation
include: extremes of weight, polycystic ovary syndrome (chronic hyperandrogenic
anovulation), emotional stress, medications, systemic illness, and structural
lesions of the hypothalamic-pituitary-ovarian axis. The initial blood
evaluation includes obtaining TSH and prolactin values routinely and an FSH if
ovarian failure is suspected. Total testosterone and DHEAS are obtained if a
hyperandrogenic condition is suspected clinically.
c) Hysterosalpingogram
In most
clinics, 30-40% of female infertility can be attributed to the pelvic
abnormalities (such as tubal occlusion, adhesions, and endometriosis). An
evaluation of the patency of the fallopian tubes involves transuterine contrast
instillation under fluoroscopic visualization. The study should be performed in
the follicular phase of the cycle prior to ovulation. Water-soluble contrast
material is generally used as it offers better detail and less risk than
oil-based contrast. An HSG should be
obtained relatively early in the infertility investigation following semen
analysis. HSG also serves to assess the contour and adequacy of the uterine
cavity. Pathology such as intrauterine leiomyomata, polyps and synechiae
(Asherman's Syndrome) and well as embryologically abnormal uterine cavities can be evaluated.
d) Postcoital Test
The couple
is asked to have intercourse at least eight hours prior to but less than 24
hours prior to presenting to clinic. Postcoital testing (PCT) should be
performed at midcycle when cervical mucus has a high water content secondary to
the midcycle estrogen surge. At other times the mucus is thick and doesn't
allow spermatozoa to penetrate. The sperm are then destroyed by the acidic
vaginal environment.
Several
characteristics will indicate whether the timing of the sample and the quality
of the mucus is good. Copious amounts of alkaline mucus are generally observed
emanating from the cervix at midcycle. A sample of cervical mucus is aspirated
and placed on a glass slide. A coverslip is applied and lifted. The degree to
which the mucus stretches (the spinnbarkeit) is measured. Columns of 8 or more centimeters is considered optimal.
Good mucus appears acellular microscopically, and when dried the high sodium
chloride content of midcycle mucus precipitates into a microscopic
fernleaf-like pattern.
In a normal
PCT, there should be at least several progressively motile spermatozoa per high
power field. If only a few dead sperm or no sperm are found, the most common
reason is a poorly timed PCT. Other reasons include oligospermia, inadequate
coital technique, hypospadias, antisperm antibodies or possibly insufficient
mucus. When sperm are seen to clump together and flagellate without progressive
motility, this is often associated with the presence of antisperm antibodies
originating either from the mucus or the semen. This test should not be used in
place of a good semen analysis. The PCT is now used sparingly due to its poor
predictive value for conception, but it remains a reasonable screen for
antisperm. antibodies.
e) Endometrial Biopsy
Approximately
one week after ovulation, the now fertilized ovum (zygote), implants. The
endometrium must be of sufficient quality to allow implantation. If not, it is
termed luteal phase inadequacy (LPI) or luteal phase defect (LPD)-- a rare
cause of infertility. Correct maturation of endometrium requires sufficient
sequential hormonal stimulation, primarily with progesterone, as well as normal
end organ responsiveness to these signals. Therefore, a single progesterone
assay alone is insufficient to diagnose LPI. Furthermore, it is important to
differentiate between inadequate luteal phase and short luteal phase. In a
short luteal phase, the duration of BBT temperature rise is less than ten days.
Shortened luteal phases are not associated with infertility.
Histological
"dating" of the endometrium. (based on the development of endometrial
glands and stroma) can assess maturation. It has become the principal means of
diagnosing LPI. Optimally, an office biopsy performed with a disposable
Pipelle' should sample endometrium from high in the uterus as soon before the
onset of menses as possible. This allows the full effect of the hormonal
stimulation during that menstrual cycle to exert its effect on the endometrium.
When the histologic appearance of the endometrium. shows a discrepancy of 2 or
more days from the norm for the day on which the biopsy was taken, then the
procedure is repeated in the subsequent cycle. Two such out-of-phase biopsies
establishes the diagnosis of luteal phase inadequacy. Due to the rarity of LPI
and the inherent inaccuracy and inconvenience of pursuing the diagnosis,
biopsies are now done only when there are risk factors for LPI such as
hyperprolactinemia, or when other infertility factors have be ruled out.
f) Laparoscopy
Diagnostic
laparoscopy is an outpatient surgical technique in which a fiberoptic scope, is
inserted through a 1 cm incision into the abdominal cavity to inspect the
pelvis under general anesthesia. A number of surgical procedures can also be
performed through accessory abdominal incisions using the scope for
visualization. Dye can be passed through a canula via the cervix transvaginally.
Patent Fallopian tubes exhibit the passage of dye through the firnbriated ends
seen laparoscopically. During the procedure a hysteroscope can also be used to
examine the uterine cavity. (This is an even more sensitive test than the
hysterosalpingograrn.)
Due to the
expense and invasiveness, diagnostic laparoscopy is reserved as the last step
of an infertility workup if no other salient etiologies are identified, and if
conservative treatments are unsuccessful. In some circumstances, a minimally
invasive laparoscopy procedure can be done under local analgesia.
REVIEW OF SECTION II
In evaluating the
causes of infertility, it is helpful to consider the reproductive system
segmentally such that no stone is unturned. Think of the hypothalamic,
pituitary, peritoneal, ovarian, tubal, uterine, and vaginal compartments as
well as systemic illnesses that can contribute to infertility. Each compartment
has its typical dysfunctions and tests.
III. TREATMENT
Goals of this
section are to understand the tools available to treat or circumvent the
specific etiologies ascertained during the evaluation.
Therapy depends
upon the etiologies identified. Anatomical defects, such as damaged tubes and
Mullerian anomalies, are corrected surgically if possible. Endocrinopathies and
systemic illnesses are treated specifically. For example, luteal phase
insufficiency may often be corrected by supplementing progesterone during the
luteal phase, and ovulation may resume spontaneously when a hyperprolactinernic
woman is treated with the dopamine agonist bromocriptine.
ANOVULATION
Approximately
10-15% of infertile females are anovulatory. If potential causes of anovulation
have been addressed and the woman remains anovulatory, attempts at medical
induction of ovulation are reasonable. Furthermore, when the etiology of
delayed fertility can not be identified after complete evaluation, and the
ovaries are known not to have undergone premature failure, ovulation induction
may also be attempted empirically ("superovulation"). The rationale
is to drive more than one oocyte to ovulate with each cycle in order to
increase the odds of a pregnancy. Likewise, intrauterine insemination of washed
sperm increases the number of male gametes potentially reaching the oocyte(s).
OVULATION-INDUCING DRUGS
A number of
medications have been used to help initiate ovulation including clorniphene
citrate, human menopausal gonadotropins (hMG), GnRH, glucocorticoids and
bromocriptine mesylate.
Clomiphene
Citrate- Relatively inexpensive, taken by mouth with few side effects (except a
multiple gestation rate of 7% in anovulatory women and the rare possibility of
inducing hyperstimulation syndrome). Requires an intact
hypothalamic-pituitary-ovarian axis. Mechanism of action is primarily within
the hypothalamus as an "antiestrogen". It occupies estrogen receptors
and "deceives" the hypothalamus into sensing a low estrogen
environment. The hypothalamus in turn signals the pituitary via pulsatile GnRH
to increase gonadotropin (FSH and LH) release to produce more follicular
development and subsequent estrogen release. With an intact
hypothalamic-pituitary axis, clomiphene has been successful in inducing
ovulation in over 90% of cases. However, pregnancy rates approach only 65 %.
Eighty percent of patients treated with clomiphene who get pregnant do so
within three cycles of therapy. Therefore judicious, limited use under
physician care is recommended. Be aware that higher doses or prolonged usage
can exert antiestrogen effects by thinning the endometriurn and thickening
cervical mucus and therefore can be counterproductive.
Human menopausal
gonadotropins, (hMG; Pergonal, Humegon, Metrodin, Fertinex Gonal-F) Extremely
expensive, given daily IM or SQ, and involves much more risk. The multiple
gestation rate is about 15-35 %, and overdosage may produce a potentially
life-threatening ovarian hyperstimulation syndrome. Therefore, close monitoring
with serial ovarian ultrasound and serum estradiol levels is necessary. hMG
consists of LH and FSH, therefore it can bypass a non-functional
hypothalamic-pituitary axis. Recombinant FSH (Gonal F) is used as above with
some risks but has no contaminating protein found in the menopausal
gonadotropins.
GnRH- Must be
given subcutaneously or IV via a continuous pump. Therefore it is cumbersome
and expensive. It requires an intact pituitary ovarian axis. It has few risks.
Glucocorticoids-
Act by suppressing ACTH and therefore adrenal androgen production. This may
occasionally be helpful in facilitating ovulation because circulating androgens
cause ovarian follicular atresia. Used primarily in polycystic ovary syndrome
with a component of elevated adrenal androgen secretion, and in women with
congenital adrenal hyperplasia.
Metformin an
insulin sensitizing agent which may be used primarily or as an adjunct to
clomiphene or hMG in insulin resistant patients with polycystic ovary syndrome.
Bromocriptine
mesylate (Parlodel)- Anovulatory women with hyperprolactinernia should be
treated initially with bromocriptine before considering ovulation induction
medications. Excess prolactin inhibits normal hypothalamic pulsatile GnRH
release.
A reasonable
strategy in some circumstances is to attempt to compel more than the usual one
oocyte to ovulate, by using ovulation induction medications. This is termed
"superovulation" and is a tactic often used for couples with
unexplained infertility and other situations of low estimated fecundity prior
to resorting to expensive artificial reproductive technologies.
ENDOMETRIOSIS
Endometriosis
is the ectopic growth of endometrium. It is diagnosed by the histologic
confirmation of both endometrial glands and stroma in an ectopic location.
Establishing
the diagnosis usually requires laparoscopy. Common symptoms may include
infertility, dyspareunia, dysmenorrhea and dyschezia. Common sites include
ovaries, broad ligament and pelvic peritoneum, cul-de-sac and bowel. The
ectopic tissue is hormonally responsive, growing in the presence of estrogen.
Sloughing endometriosis may result in pelvic lesions of a variety of colors,
older lesions appear as "gunpowder" lesions due to sequestered
hemosiderin. Endometriosis may incite inflammation with resulting adhesion
formation. Such adhesions can distort the delicate reproductive anatomical
relationships resulting in infertility. Endometriosis is clinically staged into
minimal, mild, moderate and severe categories (stages I-IV).
Although
found in approximately 5-10% of the general population, endometriosis has been
noted in 30-40% of women presenting to infertility clinics. Endometrial tissue
secretes a number of abnormal cytokines which have been implicated in aberrant
ovulation, fertilization and tubal function. Nonetheless, minimal and mild
endometriosis have not been definitively proven to cause infertility.
Both
medical and surgical techniques have been used to treat endometriosis. Only
total abdominal hysterectomy and bilateral salpingo-oophorectomy has resulted
in high rates of cure. All other treatments offer high rates of temporary
diminution of pain but should be considered suppressive only. Medical treatment
is designed to inhibit ovulation and concomitant estrogen secretion because
estrogen is "fertilizer" for endometriosis growth. A variety of
agents including oral contraceptives, progestins, GnRH agonists and danazol
have been employed for this purpose.
Ultimately,
except for mechanical restoration of pelvic anatomy, medical and surgical
treatment of endometriosis has NOT been shown to increase pregnancy rates.
Treatment may theoretically, however, slow the progression to higher stage
disease.
MALE FACTOR INFERTILITY
Unfortunately,
few cases of male factor reproductive disorders can be remedied. Evaluation for
male factor infertility is analogous to that of the female. Attention is paid
to the hypothalamic-pituitary-testicular hormonal axis (LH, FSH, testosterone),
and to the patency of the vas deferens.
Particular
abnormalities identified by semen analysis may suggest evaluation for specific
presumptive causes of male-factor infertility. For instance, elevated percentages
of tapered shaped sperm heads on morphologic examination suggest varicocele.
Varicoceles are abnormal dilatations (varices) of scrotal veins. They are
commonly found within the left scrotum superior to the testicle and feel like a
"bag of worms". They are usually caused by deficient valves in the
left internal spermatic vein and are noted in about 40% of men presenting to
infertility clinics. Treatment is by ligation of the internal spermatic vein,
but such treatment has resulted in only marginal increases in pregnancy rates
rendering varicocelectomy controversial.
Occasionally,
vas occlusion can be surgically corrected and in cases of hormonal deficiency,
replacement hormonal therapy can restore testicular function. Recent technology
allows in some instances the aspiration of sperm directly from the epididymis
in cases of blockage or absence of the vas deferens. However, the few (usually
compromised) spermatozoa obtained are usually subjected to micromanipulation
techniques during in vitro fertilization procedures so the wife must become
subjected to a procedure as well. Formerly, most cases of severe male factor
abnormalities have required consideration of donor sperm, but a dramatic
advance has been the widespread utility of a micromanipulation technique called
intracytoplasmic sperm injection (ICSI). A single motile sperm can be captured
and injected into an oocyte resulting in normal fertilization. Therefore men
with extremely low sperm counts and quality can potentially achieve a pregnancy
using their own genetic sperm.
ASSISTED REPRODUCTIVE
TECHNOLOGIES
Sperm and Oocyte
Donation- If inadequate gametogenesis is the cause of infertility, couples are
offered therapeutic donor insemination, donor oocytes, or both.
In vitro
fertilization / embryo transfer - Irreparable tubal disease requires
consideration of in vitro fertilization and embryo transfer (IVF-ET).
Endogenous gonadotropin release is first down-regulated using a GnRH agonist to
prevent interference with the ovulation induction process. Then the ovaries are
stimulated to produce maximal numbers of oocyte-containing follicles using hMG.
Oocytes are then aspirated transvaginally under ultrasound guidance during an
outpatient procedure using local anesthesia. The oocytes are incubated with sperm
in vitro. If fertilization occurs, embryos are transferred back into the
uterus. Beside tubal disease or absence, IVF-ET has been used for other
indications such as male factor infertility, endometriosis and unexplained
infertility. Success rates average 20% but some centers are now achieving >
45 % success rates ("take-home baby rate" per embryo transfer
procedure) depending on the age and infertility diagnoses of the candidates.
Zygote
Intra-Fallopian Transfer (ZIFT)- Same procedure as IVF except embryos (zygotes)
are transferred into the Fallopian tubes rather than the uterus with the
rationale that exposure to normal tubal physiology facilitates pregnancy rates.
Disadvantages include the need for normal tubes and the need for laparoscopy
under general anesthesia for tubal embryo transfer. The ability to fertilize
can be ascertained as in IVF.
Gamete
Intra-Fallopian Transfer (GIFT)- Same procedure as ZIFT except gametes (instead
of embryos) are transferred into the tubes immediately after retrieval of the
gametes. This does not allow documentation of the ability of the gametes to
fertilize.
Oocyte
cryopreservation- Unlike sperm, oocytes are extremely sensitive to
cryopreservation and do not survive the process. Once fertilization has
occurred, however, about one half of cryopreserved embryos may survive the
freeze/thaw process and can be transferred into the uterus.
Gamete
micromanipulation- When fertilization proves defective, the oocyte zona
pellucida, can be mechanically opened via micromanipulation techniques to
facilitate sperm entry. Or, a single sperm can be injected directly into the
cytoplasm of the oocyte (ICSI, intracytoplasmic sperm injection).
Ultimately, only
about 50% of couples who seek help for infertility eventually report a pregnancy.
However, amongst the group that does conceive, ectopic pregnancy, miscarriage
and perinatal mortality surpass what would be expected in the general
population. Therefore, of the original infertile population presenting for
treatment, over half will eventually confront biologic childlessness. Many
times financial and emotional stresses cause the cessation of infertility
treatments even though there may still be efficacious medical treatments
available to the couple.
A major
responsibility of the physician is to suggest to a couple when to discontinue
attempts at conception.
REVIEW OF SECTION III
Each treatment
plan must be custom designed based upon etiology(ies), patient age, resources,
desires etc. Note that available treatments are often designed to bypass
certain irreparable obstacles in the reproductive system. For example, hMG
bypasses hypothalamic/pituitary dysfunction by directly stimulating the
ovaries. IVF bypasses tubal and peritoneal disease, and optimizes sperm
function. And, artificial insemination bypasses cervical mucus dysfunction.
Understanding
the mechanisms of action of the ovulation inducing agents is critical to
rationally treat anovulatory women. There are treatments available to
circumvent almost all problems with the reproductive system with the following
three prerequisites: functional spermatozoa, functional oocytes and a normal
uterus. Even these circumstances can be skirted if the couple is willing to use
donated gametes and/or surrogacy when necessary.
IV. GLOSSARY
AI Artificial insemination
ART: Artificial
reproductive technologies (including Al, TDI, ZIFT, GIFT, IVF-ET, ED)
BBT: Basal body
temperature
Clomiphene
citrate (Clomid, Serophene): An oral antiestrogen that initiates FSH and LH
release from the pituitary
Danazol: A
synthetic testosterone derivative that prevents ovulation by inhibiting the
midcycle surge of LH, used in the treatment of endometriosis
Dyschezia: Painful bowel
movements
Dysmenorrhea: Painful menses
Dyspareunia:
Painful intercourse
ED: Embryo donation
Gonadotropins: FSH and LH
Gonadotropin
releasing hormone agonist (GnRHa): Medications which cause first stimulation
of, then downregulation of pituitary gonadotropes resulting in low FSH and LH
secretion.
hMG (human
menopausal gonadotopins: Pergonal, Metrodin, Humagon): Injectable LH and FSH
ICSI:
Intracytoplasmic sperm injection
IVF-ET: In vitro
fertilization and embryo transfer
LPD: Luteal phase
defect (inadequacy)- The presence of endometrium inadequate to support
implantation and growth
Lupron: An injectable brand
of GnRH agonist
Parlodel: Bromocriptine
mesylate, dopamine agonist that inhibits prolactin secretion
PCT: Post-coital test
Spinnbarkeit: The
stretchability of cervical mucus at midcycle
Superovulation: The use of
ovulation induction agents to purposefully ovulate more than the usual
single monthly oocyte.
TDI: Therapeutic donor
insemination
Varicocele: Abnormal
testicular vascular configuration associated with decreased sperm
GYNECOLOGY SEMINAR
CONTRACEPTION
Case
Presentations
A 20-year-old, nulligravid,
single woman has been sexually active for six months. She states that she has
been using condoms, coitus interruptus, and chance to keep from getting
pregnant. She requests an IUD for contraception because she "doesn't want
to mess with things ... and I don't remember too good."
A 31-year-old, gravida 3,
para 3 who is six weeks postpartum requests a method of contraception. She is
breastfeeding and plans to continue for one year. She requests contraceptive
advice and is considering sterilization but is unsure whether she wants to
limit her family to the three children she has.
Terminal
Objective
Given a patient requesting
contraception, the student should obtain the appropriate database and provide
sufficient information and counseling to enable the patient to choose a
satisfactory method of contraception.
Enabling
Objectives
The student should be able
to:
1. List seven methods of contraception and the effectiveness of each.
2.
Discuss
the physiologic or pharmacologic basis for each of the methods listed above
3.
List
and discuss the absolute and relative contraindications, advantages,
disadvantages and complications of each method
Definitions
Efficacy-percentage of women
experiencing an unintended pregnancy within the first year of use
Perfect use: how effective
methods can be when used consistently and correctly
Typical use: how effective methods are for the average person
Breakthrough bleeding -
nonorganic endometrial bleeding during the use of oral contraceptives. It may be
due to estrogen or progestin deficiency or missing pills.
OCP - oral contraceptive
pill, usually refers to combined oral contraceptive pill containing both
progestin and estrogen.
Contraceptives can be
divided into groups, depending on their perfect use effectiveness, their
relation to the act of intercourse, and the general approach.
1.
Efficacy. There is no perfect method of
contraception. Each patient must be
approached individually. However, the
options available to women now are vast compared to those two generations
ago. Methods differ in their
effectiveness, side effects, participation of the users, expense, availability,
ability to be concealed, and legal/religion/FDA status. Pregnancy rates are 85 to 100 woman-years
(85% per year) without contraception.
Table. Percentage of women
experiencing an unintended pregnancy during the first year of typical use and
the first year of perfect use of contraception and the percentage continuing
use at the end of the first year: United States
|
Method (1) Chance Spermicides Periodic Abstinence Calendar Ovulation Method Sympotothermal6 Post-ovulation Cap7 Parous Women Nulliparous Women Sponge Parous Women Nulliparous Women Diaphragm7 Withdrawal Condom8 Female(Reality) Male Pill Progestin only Combined IUD Progesterone T Copper T 380A LNg 20 Depo-Provera Norplant and Norplant-2 Female Sterilization Male Sterilization |
|
% of Women Experiencing an Unintended Pregnancy within the First Year
of Use Typical Use1 Perfect
Use2 (2) (3) 85 85 26 6 25 9 3 2 1 40 26 20 9 40 26 20 9 20 6 19 4 21 5 14 3 5 0.5 0.1 2.0 1.5 0.8 0.6 0.1 0.1 0.3 0.3 0.05 0.05 0.5 0.5 0.15 0.10 |
|
% of Women Continuing Use at One Year3 (4) 40 63 42 56 42 56 56 56 61 81 78 81 70 88 100 100 |
2. Coital independent - coital dependent -
coital inhibiting - postcoital
ORAL CONTRACEPTIVES
The era of modem
contraception dates from 1960 when oral contraception was first approved by the
US Food and Drug Administration, and intrauterine devices were re-introduced. A
few catastrophes attributable to oral contraceptive pills (OCP) usage,
particularly from the older higher-dose pills, caused considerable public alarm
in the 1970's. Now the birth control pill has become safer and better
tolerated, with reduced dosage of both the estrogen and the progestin
components. The 1990's are seeing multiple reports on the health benefits of
the Pill. According to the 1988 Ortho Survey, approximately 14 million women in
the United States use the pill; about 60 million women worldwide use OCPs.
("OCP" usually refers to combined
oral contraceptive pills containing both estrogen and progestin).
Few women are not candidates
to take OCPs. Table 1 lists absolute and relative contraindications. Most of
the contraindications are related to the estrogen component. Risks of hormonal
contraception must always be weighed against risks of pregnancy and
acceptability of other options. A woman is 15 to 20 times more likely to die
from continuing a pregnancy than from using oral contraceptive pills.
OCP Contraindications
Absolute Contraindications:
1. Thromboembolic disorder (or history thereof)
2. Cerebrovascular accident (or history thereof)
3. Coronary artery disease (or history thereof)
4. Impaired liver function (current)
5. Hepatic adenoma (or history thereof)
6. Breast cancer, endometrial cancer, other estrogen-dependant
malignancies (or history hereof)
7. Pregnancy
8. Undiagnosed vaginal bleeding
9. Tobacco user over age 35
10. Migraine headache with focal neurologic deficits
Relative Contraindications:
1. Hypertension
2. Diabetes mellitus
3. Surgery with immobilization (suggest 1 - 3 months
discontinuation)
4. Seizure disorder, anticonvulsant use
5. Obstructive jaundice in pregnancy
6. Gall bladder disease.
Studies to look at the
complications of oral contraceptive pills are confounded by the higher-dose
pills used in the 1960's and 1970's. Estrogen and progestin doses have been
steadily lowered, with attendant lowered morbidity. The currently prescribed
low-dose pills (<50 micrograms of ethinyl estradiol cause cardiovascular
complications (myocardial infarction, cerebrovascular accident,
thromboembolism) almost exclusively in women
over age 35 who smoke, or in some women with underlying medical problems,
particularly with conditions predisposing to thrombosis. Healthy OCP users who
undergo surgery with immobilization are at increased risk of venous thrombosis
and pulmonary embolism. Pills should be discontinued prior to surgery and
reinstated six to eight weeks postoperatively. This, too, should be balanced
against the risk of pregnancy.
Side Effects
Breakthrough bleeding is the
most common side effect for which women discontinue OCP usage. This may be due
to estrogen or progestin deficiency or to missing pills. Estrogen excess side
effects may include nausea, water retention, vascular headaches, and chloasma.
Progestin excess may lead to increased appetite and weight gain, acne,
depression, and pill amenorrhea. With current low-dose formulations, most women
experience mild or no side effects.
Benefits
Benefits of taking
contraceptive pills have been under-publicized. Long-term use is not only safe,
but it is protective against many serious disorders and nuisance complaints.
There is no need for a pill-free interval for reproductive or general health.
Noncontraceptive
Benefits of OCPs
Effective contraception
--less need for therapeutic abortion
--less need for surgical sterilization
Less endometrial cancer (50%
reduction)
Less ovarian cancer (40%
reduction)
Less benign breast disease
Fewer ovarian cysts (50% to
80% reduction)
Fewer uterine fibroids (31 %
reduction)
Fewer ectopic pregnancies
Fewer menstrual problems
--more regular
--less flow
--less dysmenorrhea
--less anemia
Less salpingitis (pelvic
inflammatory disease)
Less rheumatoid arthritis
(60% reduction)
Increased bone density
Probably less endometriosis
Besides providing protection
from the above medical disorders, OCPs are used to manage many gynecologic
disorders.
Noncontraceptive
Uses of OCPs
Definitely beneficial:
--Dysfunctional uterine bleeding
--Dysmenorrhea
--Mittelschmerz
--Endometriosis prophylaxis
--Acne and hirsutism
--Hormone replacement
--Prevention of menstrual porphyria
Beneficial in many cases:
--Functional ovarian cysts
--Premenstrual syndrome
--Control of bleeding (dyscrasias, anovulation)
The OCP has over 25
preparations, using two different estrogens and several different progestins.
The available pills contain fixed and variable-dose ratios. All can claim >
99 % theoretical effectiveness. Combination pills, using both estrogen and
progestin, are taken for 21 days, with a seven-day hiatus between cycles,
during which time withdrawal bleeding occurs. The "minipill", or
progestin-only pill, is taken continuously without a break; bleeding may occur
irregularly, not at all, or occasionally as regular menstrual cycles.
Additional information can be found under "progestins".
The principle mechanisms of
action of OCP's appear to be:
1. Blockage of ovulation, which is mediated through hypothalamic suppression of FSH, and the LH surge.
2. Creation of "hostile" (viscid) cervical mucus to
hamper the transport of sperm and decrease sperm penetration.
3. Prevention of
implantation by altering the endometrium so that it is not receptive to the
blastocyst.
4. Other probable factors of decreased tubal transport and sperm
capacitation.
Choosing an oral
contraceptive is simpler than it may seem. All pills protect against pregnancy
in most women. Start with a preparation containing 30 or 35 mcg of ethinyl
estradiol. The newer" progestins, norgestimate and desogestrel, are
reported to have equal progestin but less androgen effect than the traditional
progestins (norethindrone, levonorgestrel, etc.). For new starts, ethinyl
estradiol-plus-norgestimate (Ortho-Cyclen, Ortho Tri-Cyclen) may be the best
option, per the theory that less androgen effect will be better for the
cardiovascular system. Older low-dose oral contraceptive pills which have been
well studied and proven safe and effective, are also recommended (Norinyl 1+35,
Ortho-Novum 7-7-7, Demulen 1/35, Ovcon 35, Loestrin 1.5/30, etc.). One study
reported that desogestrel-containing oral contraceptive pills's (OrthoCept,
Desogen) cause a slightly higher incidence of thrombotic events. These data
have not been corroborated, and these oral contraceptive pills's do not need to
be discontinued but are not recommended for new starts. All tri-phasic pills
have the same amount of estrogen throughout the month, but varying doses of
progestin. This is formulated to provide less total monthly progestin exposure,
theoretically enhancing cardiovascular health.
New Methods of Administration of Combination Estrogen/Progestin for Contraception
Within the past 2 years,
there have been new methods of administering estrogens and progestins for
contraception. In an effort to increase
compliance and decrease the need for daily administration of an oral pill, new
products have been introduced to try to lower the user-failure rate of
combination hormonal contraception:
Contraceptive transdermal patch changed
weekly, administered for three weeks with one week off
Contraceptive
vaginal ring an intravaginal ring which releases continuous estrogen and
progestin which is changed monthly (three weeks of ring use with one week off)
Women may discontinue usage for side effects such as breakthrough bleeding, amenorrhea or nausea, or for side effects with possible relationships to pill use such as weight gain, headaches or acne. Many women in the United States are unable to pay for contraceptives, and instead find themselves dealing with the much more expensive problem of childrearing. Better education improves compliance. A patient should know when to contact her physician, with the potential danger signals listed below. She should also be thoroughly informed about the safety of the pill and of its benefits.
EARLY PILL DANGER SIGNS
A Abdominal pain (severe)
C
Chest pain (severe), cough,
shortness of breath
CAUTION H Headache (severe), dizziness, weakness, or numbness
E
Eye problems (vision loss or
blurring), speech problems
S
Severe leg pain (calf or thigh)
See your clinician if you
have any of these problems, or if you develop depression, yellow jaundice or a
breast lump.
Progestin Administration
Progestins can be
administered on a continuous basis in oral (progestin only pill), or injectable form (Depo-Provera). Progestin only contraceptives work by inhibiting
ovulation, thickening cervical mucous, and causing atrophy of the endometrial
lining. The main drawback to all these
methods is unpredictable, irregular bleeding in many users. The advantages of the injectable form is effectiveness, lack of use
responsibility, and lack of impact on lactation. In comparison to the combination birth control pill, the
progestin only birth control pill is less effective (except when combined with
lactation), associated with more break through bleeding, and fewer serious side
effects. Women must be compulsive about
taking the pill at the same time each day for maximum efficacy. This is no pill free interval.
Health concerns revolve
around changes in lipid levels. In general, some lowering of HDL (high-density
lipo-protein, the "good" cholesterol) can be measured, but this has
not been shown to contribute clinically to heart disease. Progestins do not
promote clotting, therefore, they do not increase the risks of heart attack or
stroke regardless of age or smoking status. Despite this, the FDA requires the
same thrombosis precautions on all hormonal contraceptives because of the
technical approval process.
Table 3. Brand Names of
Progestin-Only Pills
|
Progestin |
Dose (mg) |
Number of Tablets Per Package |
Brand Names |
|
Norethindrone
(Norethisterone) |
350 |
42/28 |
Micronor, NOR-QD Noriday, Norod |
|
Norethindron
(Norethisterone) |
75 |
35 |
Micro-Novum |
|
Norgestrel |
75 |
28 |
Ovrette, Neogest |
|
Levonorgestrel |
30 |
35 |
Microval, Noregeston,
Microlut |
|
Ethynodiol
diacetate |
500 |
28 |
Femulen |
|
Lynestrenol |
500 |
35 |
Exluton |
Depo-Provera is
medroxyprogesterone acetate in a sustained-release suspension, 150 mg IM every
three months. It is extremely effective with a failure rate of 0.3% during the
first year of use. Fifty percent of women
using depo-provera will experience amenorrhea after one year of use. The most common side effects are irregular
bleeding and a slight increase in weight.
The average return to fertility is 9-10 months.
.
Intrauterine
Devices
Intrauterine devices are
plastic, polyethylene devices impregnated with barium sulfate to make them
radiographic, now containing copper or progesterone, which stay in the uterine
cavity. They cause a sterile spermicidal inflammatory reaction. Very few sperm
reach the oviducts, and fertilization usually does not occur. If it should
occur, the inflammatory reaction is also toxic to the blastocyst, and
implantation is prohibited. There are two IUD's currently marketed in the
United States. "ParaGard" (TCu-380A) is a copper-containing IUD with
an efficacy lasting ten years. Mirena is a progestin-releasing IUD which must
be replaced every 5 years.
First-year IUD failure rates
range from < 1 % to 3.7 %. Pregnancy usually follows spontaneous expulsion
of the IUD, occurring most commonly shortly after insertion. Cumulative four to
six year pregnancy rates are less than 1 % per year. An experienced clinician
has fewer failures, due mainly to correct high-fundal insertion.
The advantage of the IUD
include its use in women unable to take estrogen, lack of systemic side
effects, immediate high efficacy, a rapid return to fertility after removal,
the necessity for a single motivational act, and a lack of interference with
lactation. The disadvantages are a
slight increased risk of infection during the month following insertion, an
increase in menstrual bleeding and cramping with the copper IUD (the progestin
IUD decreases blood loss and dysmenorrhea), the rare complications of expulsion
and perforation.
Pregnancies may occur with
the IUD in place. This carries about a 55 % risk of spontaneous abortion.
Removal of the IUD after pregnancy is diagnosed, lowers this risk to about 25
%. There may be a higher risk of septic (spontaneous) abortion, but with modern
IUD's this is not certain. IUDs
substantially decrease the risk of ectopic pregnancies compared to not using
contraception, but they are more effective at preventing intrauterine than
preventing ectopic pregnancies. If a
woman using an IUD does become pregnant, she has a higher chance of having an
ectopic pregnancy (3-4% with the copper IUD 1.5% in the general population).
Concerns regarding the
safety of the IUD relate mostly to pelvic inflammatory disease (PID) and
subsequent infertility. These concerns
are largely unwarranted with modern IUD's. The Dalkon Shield was found to be
the main offender and was removed from the market. Other IUD's were removed
from the market because of the cost of defending against multiple malpractice
suits. The incidence of PID in monogamous IUD users is slightly greater than
background risk only shortly after insertion. Exposure to more than one sexual
partner greatly increases infectious risks. The ideal patient for an IUD is a
monogamous woman who thinks she may be finished with childbearing.
Contraindications for IUD
use include:
-Known for suspected pregnancy
-Active, recent (three months), recurrent pelvic infection
including postpartum endometritis,
septic abortion, PIH,
cervicitis
-Distorted uterine cavity from leiomyomata, uterine anomalies,
etc.
-Risk factors for infection including multiple
partners, history of recurrent sexually transmitted
infections
-Impaired immune response from chronic steroid use, HIV, etc.
-Undiagnosed abnormal vaginal bleeding
-Known or suspected cervical or uterine malignancy, including an
unresolved abnormal PAP smear
-History of pregnancy, perforation with prior IUD
In addition, the copper IUD
should not be used in women with a copper allergy or Wilsons disease. The progestin containing IUD (Mirena, containing
levonorgestrel) should not be used in a woman with a previous adverse reaction
to levonorgestrel).
Surgical Sterilization
Surgical sterilization is
increasing in popularity as a form of contraception. 10.7 million women in the United States rely on female
sterilization, 4.2 million on vasectomy.
Bilateral tubal ligations
may be performed postpartum using the Pomeroy, Parkland, Uchida, or Irving
techniques through a small infraumbilical incision. Interval bilateral tubal ligations are usually performed with the
laparascope using electrocautery, silastic bands, or spring clips.
The U.S. Collaborative review of Sterilization
(CREST) study is the largest U.S. study on female sterilization. It reports a 10-year cumulative failure rate
of 18.5 pregnancies for every 1000 procedures.
This is higher than previously thought.
Failures may occur as long as a woman is fertile, not just in the first
1-2 years following the procedure.
Failure rates were higher in young women and with specific methods (spring
clips and bipolar electrocautery compared to postpartum sterilization). However, sterilization remains the most
effective method of contraception (regardless of technique) for women >34
years. It is important to note that
longterm hormonal contraceptive methods (implants and Depo-Provera) and IUDs
have annual failure rates of about 2/1000 very close to the failure rates of
sterilization.
The advantages of
sterilization include permanence, effectiveness, lack of side effects. The disadvantages are surgical risk
(mortality 1-2/100,000), risk of regret, risk of ectopic if pregnancy should
occur.
A bilateral tubal ligation
protects against ectopic pregnancy compared to not using contraception. However, in the few pregnancies which do
occur, there is an increased risk (33% compared to 1.5% in the general
population). Regret occurs more often
in young patients, in postpartum or postabortion tubals, and when patients
life situation changes. Tubal reversal
is expensive and successful in only 43-80% of cases. In vitro fertilization is a very successful options for young
women who with a child after tubal ligation (about 50%/IVF cycle) but is quite
expensive. Therefore, a patient should be certain that she doesnt want more
children prior to having a bilateral tubal ligation.
Vasectomy is safer, less
expensive, and more effective than female sterilization. The one year failure rate is 0.15% compared
to 0.5%. The cost is about 1/3 that of
bilateral tubal ligation. Vasectomies
are performed in the office with local anesthetic. Two semen analyzes must be negative for sperm (approximately
15-20 ejaculations) after the procedure.
Fertility Awareness Methods
Fertility awareness methods
are based upon identifying the days in each menstrual cycle in which
intercourse is most likely to result in a pregnancy. This is termed fertility awareness combines methods (FACE) if
pregnancy is avoided by using barrier methods or coitus interruptus. Natural family planning (NFP) refers to
abstaining from intercourse during fertile days. Typical use failure rates are 25% during the first year. With perfect use failure rates of 1-9 %
occur.
Four indicators may be used
to predict periods of fertility.
1. Cervical secretions increase and become clear and stretchy near
ovulation.
2. The cervix itself, becomes softer and wider near ovulation.
3. Basal body temperature (BBT) rises under the influence of
progesterone after ovulation
4. Calendar calculations may be made based on the length of a womens
menstrual cycle.
Most couples initially
require an instructor to help interpret signs of fertility. Fertility awareness methods are more
difficult to interpret if a women has recently been on a hormonal form of
contraception, if she is near menarche or menopause, or is he is recently
postpartum or breastfeeding.
Barrier Methods
Barrier methods of
contraception include the condom, diaphragm, cervical cap, and vaginal
spermicides.
The male condom offers the
most effective method of preventing sexually transmitted infections. Male condoms are manufactured from latex,
lamb caecum, or polyurethane. All
prevent pregnancy. Naturally membrane
condoms do not offer the same protection against sexually transmitted
disease. Small pores may permit the
passage of viruses, including HIV, hepatitis B, and HSV. Polyurethane condoms may be used for
patients with latex allergies. With
latex condoms, only water-based lubricants (KY jelly, spermicidal agents)
should be used. Oil based lubricants
(lotion, petroleum jelly, massage oil) may damage the condom. The failure rate with condom use the first
year is 3% with perfect use and 14% with typical use. Failures occur more commonly because condoms are not used with
every act of intercourse, rather than from slippage or breakage. The advantages of condom use include
protection from STDs, low cost accessibility, and lack of side effects. All patients at risk for sexually
transmitted infections should be counseled to use condoms. The first female condom, call Reality, was
approved by the FDA in 1993.
Vaginal spermicides are used
with the diaphragm and cervical cap.
They may be used alone, but have a wide range of failure rates, from
5-50% in the first year. Spermicides consist
of a base (gel, foam, cream, film, suppository) and an active chemical agent
(nonoxynol-9) in the United States).
Suppositories and film must be placed at least 15 minutes before
intercourse to allow adequate dispersion.
Spermicides might slightly decrease the risk of sexually transmitted
infection (by approximately 25%). The
advantages of spermicides are their accessibility, ease of use, and ability to
augment other forms of contraception.
They are not good options if a patient is allergic to the base or
spermicidal agent or if she has abnormal vaginal anatomy (such as a septum).
The diaphragm is a dome
shaped cup that rests between the pubic symphysis and in the posterior
fornix. Diaphragms are manufactured in
different sizes, from 50 mm to 95 mm. A
patient should be fitted with the largest size that is comfortable. The diaphragm is used with a spermicidal
cream or jelly. It may be placed up to
6 hours before intercourse, should be left in place for at least 6 hours before
intercourse, and should not be worn more than 24 hours total. A diaphragm should be refitted after a
weight gain or loss of 10 pounds and postpartum. The diaphragm has a 20% failure rate with typical use, a 6%
failure rate with perfect use over the first year.
The cervical cap is as
smaller that fits snugly over the cervix.
The cervical cap is manufactured in 4 sizes. Because of the limited number of sizes, 6-10% of women are unable
to be fitted properly. The cap my be
left in place for up to 48 hours total, and should be left in place for at
least 6 hours after intercourse. It
also is used with a spermicidal preparation.
The cervical cap is more efficacious in nulliparous women. The failure rate is 20% for typical use, 9%
for perfect use in nulliparous women.
In parous women the failure rate is 40% for typical use, 26% for perfect
use.
The advantages of the
diaphragm and cervical cap include a lack of systemic side effects and
potential protection against STDs. They
are good choices for women who need contraception intermittently. They should be used with caution in women
who have allergies to latex or spermicides, vaginal anatomy abnormalities, a
history of Toxic Shock Syndrome, or recurrent urinary tract infections.
Coitus Interruptus (Withdrawal)
Coitus interruptus is used
as the primary means of contraception by at least 2% of couples in the United
States. In some countries, it is the
most commonly used form of contraception.
With perfect use there is a 4% failure rate during the first year, with
typical use 19%. This is similar to
barrier methods. Its advantages are
its cost and lack of side effects. It
is unforgiving if used inconsistently and does not offer STD protection.
Postpartum Contraception
Breastfeeding women will
have a longer period of postpartum infertility than will nonbreastfeeding women. Women who solely breast-feed who continue to
experience amenorrhea are 98% protected from pregnancy in the first six months
following delivery. In nonlactating
women, the first ovulation occurs on average 45 days postpartum. Good contraceptive options for lactating
women include barrier methods (the diaphragm and cervical cap should be refit
at six-weeks postpartum), progestin only methods, the IUD (may be placed
immediately postpartum, but more often is placed at 6-8 weeks postpartum), and
male or female sterilization. The
combination birth control pill is not a good option because estrogens decrease
milk supply but may be given after 3 months when the milk supply is well
established. In women who are not
breastfeeding, the combination birth control should not be started until 2-3
weeks postpartum because the risk of thromboembolism is increased during this
time. Fertility awareness methods may
be difficult to practice until regular cycles are established.
Emergency Contraception
Emergency contraception is
defined as methods women may use after intercourse to prevent pregnancy. The available regimens include combination
oral contraceptives, oral progestin, and the IUD. Two tablets of Ovral, or four tablets of Nordette, Levelen,
Lo/Ovral, Triphasil, or Tri-Levlen taken as two doses, 12 hours apart, within
72 hours of unprotected intercourse. Preven is a product with ethinyl
estradiol and levonorgestrel which is specifically approved for emergency
contraception. This reduces the risk of
pregnancy by 74%. Nausea and vomiting
are the most common side effects. Women
should be counseled about STD screening, contraceptive options (emergency
contraception is not as effective as other methods), and the need to check a
pregnancy test if there is a delay in menstruation. The IUD may be inserted within five days of unprotected
intercourse. A progestin only regimen
of two doses of levonorgestrel (0.75 mg) given 12 hours apart is also
effective, with less nausea than the combined oral contraceptive regimens.
Plan B is a product with levonorgestrel which is specifically approved for
emergency contraception.
Major
Take Home Points
1. Surgical sterilization of women and oral contraceptive use by
women are the most common methods of contraception in the U.S., and are some of
the most effective methods.
2. Contraindications
to combined estrogen/progestin OCP use are thromboembolic disorders,
cerebrovascular accidents, coronary artery disease, liver abnormalities,
estrogen dependent cancers, pregnancy, undiagnosed vaginal bleeding and tobacco
use over age 35.
3. Noncontraceptive benefits of combined oral contraceptives
include decreased endometrial cancer, uterine cancer, benign breast disease,
ovarian cysts, uterine fibroids, ectopic pregnancy, menstrual irregularities,
salpingitis, rheumatoid arthritis, endometriosis, atherosclerosis, and
increased bone density.
4.
Intrauterine
devices are safe and effective contraceptive methods especially for monogamous
females near the end of their reproductive careers.
5.
Barrier
methods and rhythm methods are highly dependent on the individuals involved.
6.
Pregnancies
in women who have undergone a surgical sterilization should be considered
ectopics until proven otherwise. Likewise, a positive pregnancy in a woman with
an IUD may be an ectopic pregnancy.
7.
Good
contraceptive options for lactating women include barrier methods, progestin
only methods, the IUD, and sterilization.
8. Emergency contraception reduces the risk of unintended pregnancy
by at least 74% after unprotected intercourse or contraceptive failure.
References
1. Hatcher RA, et al. Contraceptive Technology 1990-1992, 16th
revised edition. New York: Irvington Publishers, 1994.
2. Mishell DR Jr., Davajan V, Lobo RA. Infertility, Contraception
and Reproductive Endocrinology, 3rd edition. Boston: Blackwell Scientific
Publications, 1991.
3. Speroff L, Darney P. A Clinical Guide for Contraception.
Baltimore: Williams & Wilkins, 1996.
Sexually
Transmitted Diseases
Paul R.
Summers, M.D.
Department of Obstetrics
and Gynecology
Without exception, the spread of disease
during sexual contact requires a sequence of events that involve the infectious
source, as well as the new host.
Exposure to a critical number of microbes is an essential requirement
before disease transmission can occur.
To invade through the protective layer of the stratum corneum, these
microbes first must be able to adhere to the exposed epithelium. Adherence requires the presence of specific
binding sites that are generally native protein sequences within the skin. Skin microtrauma during sexual relations may
compromise the skin barrier by increasing the number of exposed binding
sites. The integrity of the local skin
barrier and mucosal immunity act as the chief defenses against the sexual
transmission of infection. Failure of
this defense system in the genital area allows sexual transmission of disease.
I. What is a Sexually Transmitted Disease
A wide variety of microbes are
transmitted by sexual contact. The
majority of these microbes can colonize the genital area, but do not cause
disease. A disorder attributed to one
of the limited number of microbes that can produce disease is termed a sexually
transmitted disease (STD). To be
considered an STD, the origin of the infectious microbes as well as the site of
microbial invasion in the new host must be the reproductive organs.
Contrary to reasonable expectations, STD
transmission usually does not result in symptomatic disease. Thus, many more individuals are STD carriers
than symptomatic cases. This frequent
delay or failure to develop symptoms is problematic for STD prevention and
control. Most infected individuals are
not aware of their STD, do not seek treatment, and may spread the infection
unknowingly.
Factors that influence development of symptomatic disease are
only partially understood. Herpes
simplex infection is likely to remain unrecognized because of decreased visibility
of the lesions if the vagina or cervix is the primary site of infection. Trichomonas infection is typically
symptomatic in the female, but asymptomatic in the male. This may be due to a smaller number of the
estrogen-dependent trichomonas binding sites in the male genital epithelium. In contrast, there is no explanation why chancroid
is generally less severe for the female and is diagnosed 10 times more
frequently in the male.
II. Who is at Risk
The physical contact required for
reproduction provides an opportunity for transfer of microbes. Any life form that replicates sexually is
then theoretically at risk for STDs.
Table I gives some examples of non-human STDs.
